Abstract
Abstract  2107
2107
Iron deficiency is the most common cause of anemia worldwide affecting 50% of children under 5 years of age and 25% of women under the age of 50 (11% in the USA) worldwide. Standard treatment is oral iron supplementation, however this route of administration is associated with several adverse drug reactions (ADRs), the most common being epigastralgia and constipation leading to lack of compliance or dose reduction in 30% of patients. Intravenous (IV) iron is an alternative treatment for patients intolerant or non-responsive to oral formulations. Of the two most common formulations available in Canada, IV iron dextran is less expensive but may be associated with more overall ADRs compared to IV iron sucrose.
We conducted a single centre, double-blinded pilot RCT to assess the feasibility of a full RCT to compare ADRs between iron dextran and iron sucrose in non-hemodialysis and IV iron treatment naïve adult patients with iron deficiency anemia. The incidence of immediate (during outpatient hospital visit) and delayed (within 24 hours after patient discharged) ADRs for each iron formulation were compared. Baseline characteristics of participants were analyzed by means of descriptive statistics. Demographic and clinical characteristics of study participants were evaluated by adverse reaction status. They were compared using a two-sample t-test for continuous variables and a two-way contingency table using Chi square or Fisher's exact test for categorical variables, as appropriate. Patients were contacted 24 hours after discharge to answer a standardized questionnaire. Assessment of ADRs and severe ADRs were done using the standardized World Health Organization and International Conference on Harmonisation definitions. Grading of severity was done independently by three individuals from an independent Drug Safety Monitoring Board.
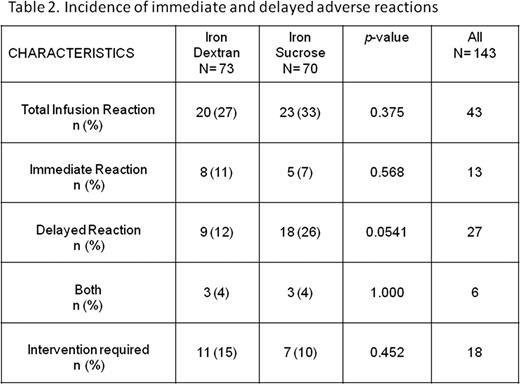
143 adult patients with iron deficiency anemia were were approached and erolled in the study between January 2008 and January 2009 (100% enrolment). Baseline characteristics of patients is depicted in Table 1. All patients received IV iron (73 iron dextran and 70 iron sucrose) and none were lost to follow-up. Immediate ADRs were similar between the two study arms; iron dextran 8/73 (11%) and iron sucrose 5/70 (7%), p=0.568. The average additional nursing time required to manage immediate ADRs was approximately 30 minutes. Delayed ADRs approached statistical significance with a higher rate of ADRs in the iron sucrose group [ iron dextran 9/73 (12%) and iron sucrose 18/70 (26%), p=0.0541]. Details in Table 2. Four patients were considered to have serious adverse reactions (shortness of breath, bronchospasm and diaphoresis). Two form each study arm.
A RCT to compare adverse drug reaction rates between iron dextran and iron sucrose in non-hemodialysis adult patients with iron deficiency anemia is feasible. In our pilot study we were able to get 100% enrolment rate, in a timely fashion with no patients lost to follow up. The design of the study with a one point in time evaluation and a short follow up that did not require extra hospital visits and blood tests were probably attractive features that maximized patient participation. The incidence and severity of ADRs to both IV iron preparations studied were similar with greater than 25% of patients experiencing either immediate or delayed ADRs. However, a striking higher rate of delayed ADRs, albeit not severe, was slightly more pronounced in patients receiving iron sucrose therapy. The elevated level of ADRs to IV iron suggests the need for alternative formulations and a full RCT to compare the rate of ADRs between iron preparations is warranted. The choice of IV iron formulation in the adult non-hemodialysis population should take into account factors other than cost of the medication such as incidence and severity of ADRs, extra time required by Healthcare professionals, and patient preferences.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal