Abstract
Abstract 2006
Allogeneic hematopoietic stem cell transplantation (HCT) is an effective treatment for patients with advanced myelofibrosis. Myeloablative regimens have produced a high rate of morbidity and mortality. Reduced intensity conditioning (RIC) with allogeneic transplantation is being investigated with the goal of reducing non-relapse mortality. There is concern that this benefit might occur at expense of a higher relapse rate. In 2006 we initiated a study of RIC regimen of fludarabine 40mg/m2 (days −5, −4, −3 −2) and IV busulfan 130mg/m2 (day −3,−2). In the first 14 patients (the Bu-low group), 9 patients relapsed. We hypothesized that high dose PK guided IV busulfan would decrease relapse rate without substantially increasing non relapse mortality, thereby improving event free survival (EFS). The next 19 patients received an increased intensity of busulfan (Bu-high group), using pharmacokinetic dose adjustment.
Patients with intermediate or high risk MF were eligible if they had adequate organ function and at least 9/10 matched related or unrelated donor. Patients received IV busulfan dose to a target daily AUC of 4000 μmol/L × 4days (days −5, −4, −3 −2) Fludarabine 40mg/m2 (days −5, −4, −3 −2). Average busulfan dose in Bu-high group was 473 mg/m2 (range 304–886 mg/m2) or 10.8 mg/kg, and it was 260mg/m2 or 6.5mg/kg in Bu-low group. Both cohorts of patients received tacrolimus and mini methotrexate as graft versus host disease prophylaxis and similar supportive care. Thymoglobulin 2.5 mg/kg × 3 (day −3,−2 and 1) was given to all patients receiving an unrelated donor graft.
Between 1/2006 and 12/2010, 33 consecutive patients with myelofibrosis were treated including 14 in the Bu-low and 19 in the Bu-high groups. Patients and disease characteristics were similar for the two treatment groups. There were 16 males and 17 females with a median age of 59 (range 27–70). Eighteen patients had primary MF, 8 post PV MF and 7 Post ET MF. Based on Lille criteria, 17 patients had intermediate risk disease and 16 had high risk disease. Eleven patients had splenectomy prior to transplant; remaining 22 patients had enlarged spleen at the time of transplant. Twenty patients had mutated Jak 2. Median peripheral blood CD 34 count was 54/μl (range 1–16426/μl). Karyotype was abnormal in 12 patients and diploid in 21. Donors were matched siblings for 12 patients, matched unrelated for 17, and one antigen or allele mismatched unrelated for 4 patients. Stem cell source was marrow in 4 and peripheral blood in 29. Circulating blasts were present in 17 patients.
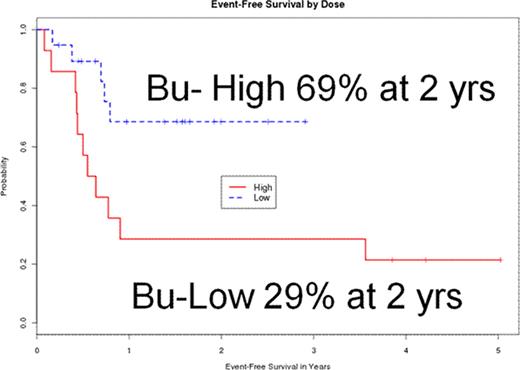
All patients engrafted with a median time to neutrophil engraftment of 13 days (0–27) days and a median time to platelet engraftment of 18 (0–105) days. Cumulative incidence of acute GVHD was 28% and chronic GVHD 35% (limited 7%, extensive 28%). Cumulative incidence of non relapse mortality was 10% with overall 3 treatment related deaths: 1 in Bu-high group and 2 in Bu-low group (p=0.44). With a median follow up of 1.6 (0.2–5) years, current EFS (p=0.02) and the cumulative incidence of relapse (p=0.04) were significantly different between Bu-high and Bu-low groups, while overall survival (OS) was not (0.28). Two year estimates for the Bu-high and Bu-low groups are as follows: EFS: 69% and 29%; OS: 87% and 71%; cumulative incidence of relapse: 26% and 57%. Multivariate analysis showed that Bu-high dose (HR 0.25; p=0.01) and peripheral blood CD 34 count >100 μl (HR 3.56; p=0.02) were significant predictors of EFS.
Higher dose busulfan, delivered with pharmacokinetic dose adjustment, reduces relapse without increasing non relapse mortality, resulting in better EFS in patients with MF.
Popat:Otsuka: Research Funding. Andersson:Otsuka American Pharmaceuticals, Inc.: Consultant for Clinical Protocol Development. Nieto:Otsuka: Research Funding. Qazilbash:Otsuka: Research Funding. Champlin:Otsuka: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal