Abstract
Abstract 1987
MultiStem®, a multipotent adult progenitor cells (MAPC) collection, is an immune-modulatory, bone marrow-derived adult adherent allogeneic stromal cell product manufactured to large scale, assuring consistency of universal donor cell product during clinical evaluation. Pre-clinical studies showed safety of IV infusion and survival benefit in a haploidentical transplantation rat model of acute GVHD (Kovacsovics 2008, 2009).
An open label Phase I clinical dose escalation study is being conducted with the primary goal to assess safety of MAPC as an adjunct treatment for human subjects undergoing myeloablative allogeneic HSCT for hematologic malignancies. Patients have been enrolled for MultiStem® administration as a single dose or in multiple weekly doses. Infusional toxicity and RRTs are assessed for 30 days following the last study drug dose administration. Secondary endpoints include incidence of acute GVHD, infection and survival through day 100. Dose escalation is guided by the Continual Reassessment Method (CRM).
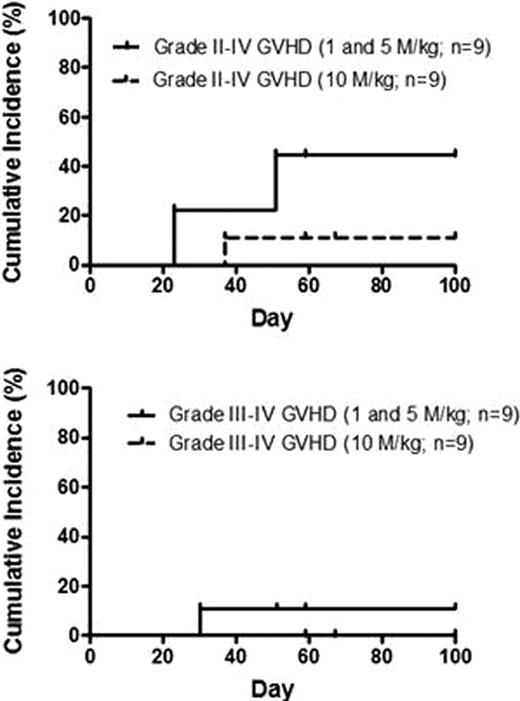
In the single dose arm, 18 patients with AML (9), MDS (4), CML (3) and ALL (2) from 5 clinical centers were administered MultiStem® IV at 1, 5, or 10 million cells per kg at day 2 after allogeneic HSCT. There was no observed infusional toxicity. Two patients experienced Bearman RRTs (Gr 3 mucositis; Gr 3 renal & pulmonary failure) deemed unrelated to study product. Engraftment occurred in all 18 patients. The median time to neutrophil engraftment for related (n=7) and unrelated (n=11) HSCT was 15 days (range, 12–16 days) and 14 days (range, 11–25 days), respectively. The 100-day cumulative incidence of Grade II-IV and III-IV GVHD was 28% and 6% (n=18). In the highest MultiStem® dose group (n=9) there was no grade III-IV GVHD, and only one case of grade II GVHD, which subsequently resolved with treatment. 17/18 patients have been enrolled in the multidose arm. Results of safety endpoints and interim results of all secondary endpoints of repeated MultiStem® administrations following HSCT will be presented.
Single dose administration of MultiStem® is well-tolerated, without observation of infusional toxicity or graft failure. Together with the observed low incidence of severe aGVHD, these findings are supportive of the concept that stromal stem cell therapy is safe and may be harnessed as an efficacious therapeutic option for acute GVHD prophylaxis following HSCT.
Cumulative incidence of acute GVHD after administration of MAPC on Day 2 after conventional allogeneic HSCT in setting of tacrolimus & methotrexate GVHD prophylaxis.
Cumulative incidence of acute GVHD after administration of MAPC on Day 2 after conventional allogeneic HSCT in setting of tacrolimus & methotrexate GVHD prophylaxis.
Maziarz:Athersys, Inc: Patents & Royalties, Research Funding. Perry:Athersys, Inc.: Employment. Deans:Athersys, Inc: Employment. Van't Hof:Athersys, Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal