Abstract
Abstract 1895
One of the major limitations of allogeneic hematopoietic stem cell transplantation (allo-HSCT) is graft-versus-host disease (GVHD), which is a severe and common complication that impacts on multiple organ systems with varying degrees of severity. Neurological complications such as encephalopathy, meningoencephalitis, cerebrovascular disease, demyelination and in particular neurocognitive deficits, have been reported in patients after allo-HSCT but their etiology remains in most cases poorly understood. In this study we analyzed whether the central nervous system (CNS) is a target of acute GVHD.
We used a murine MHC-disparate allo-HSCT model to study cerebral involvement in GVHD: C57BL/6 (B6) (H-2b) into lethally irradiated (850 cGy, split dose) BALB/c (H-2d). The control group received a syngeneic BMT (syn-HSCT) with BALB/c into BALB/c.
CNS infiltrating lymphocytes were isolated from brains after complete perfusion of the animals. We found increasing numbers of CNS-infiltrating donor derived CD4 and CD8 alloreactive T cells on day 7, 14, and 21 (p<0.001) in allo-HSCT recipients, but almost none in syn-HSCT controls.
In addition, in order to assess cytokines and homing molecules necessary for CNS infiltration in GVHD, we performed competitive BMTs by mixing control B6 (CD45.1) with either WT B6, IL17−/−, IL21R−/−, or L-selectin−/− (all CD45.2) in a 1:1 ratio. We found statistically significant and reproducibly decreased numbers of infiltrating T cells using IL21R−/− donor T cells, which we have recently reported to mediate decreased systemic GVHD and gastrointestinal infiltration as well.
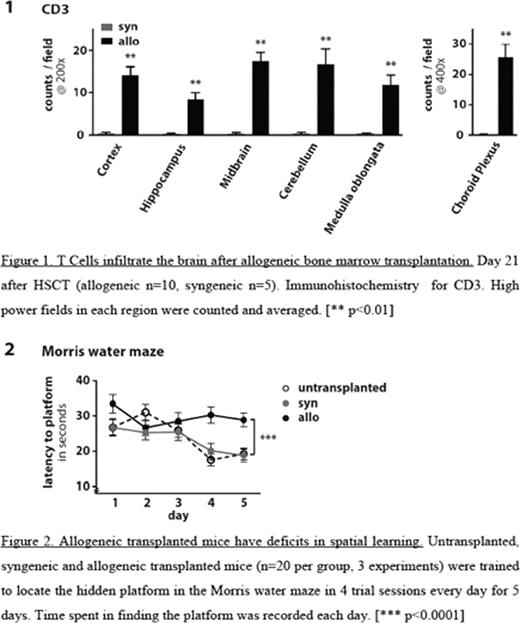
To confirm the cerebral involvement in situ, histological analysis and immunohistochemistry for CD3, CD4 and TUNEL (Terminal deoxynucleotidyl transferase mediated dUTP Nick End Labeling) on coronal brain sections from perfused animals were performed. We observed infiltration of T cells in Cortex, Hippocampus, Midbrain (Basal ganglia, Thalamus, Hypothalamus), Cerebellum, and Medulla oblongata of allo-HSCT recipients, which was consistent with lymphocytic multifocal meningoencephalitis. Sections from syn-HSCT recipients showed no pathology and T cells were virtually undetectable (p<0.01). Apoptotic cell death by TUNEL staining was only observed in allo-HSCT recipients.
To test whether these histopathological findings were associated with cognitive deficits, we performed behavioral tests at 14 to 20 days after HSCT: Locomotor activity was evaluated in the accelerated rotorod (latency to fall) and open field (total distance moved) tests; neuromuscular function and strength were tested in the grip test (latency to fall); anxiety-related behavior and exploratory activity were tested in the open field (distance moved/time spent in center) and in the elevated plus maze (time spent in open arm); and spatial learning and memory were analyzed in the Morris water maze. Although allo-HSCT recipients had developed mild to moderate GVHD in the first three weeks after transplant as measured by GVHD score and weight loss, we found no differences between allo-HSCT versus syn-HSCT recipients or non-transplanted mice in locomotor activity, motor coordination, strength and neuromuscular function. However, allo-HSCT recipients demonstrated increased anxious behavior and decreased exploratory activity in the open field test (p<0.01). Importantly, spatial learning was impaired in allo-HSCT recipients as compared to both syn-HSCT recipients and non-transplanted controls (p<0.0001). Of note, both the decreased exploratory behaviour and the spatial learning defect were not due to a lack of mobility of allo-HSCT recipients, because the allo-HSCT recipients covered similar distances in both tests as the syn-HSCT recipients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal