Abstract
Abstract 1786
The Wnt/beta-catenin pathway is highly active in chronic lymphocytic leukemia (CLL) and confers an anti-apoptotic effect in vitro and possibly also in vivo. As such, inhibition of this pathway represents a potential therapeutic target.
Here we report preclinical studies using agelastatin A (AgA), a naturally occurring alkaloid extracted from the marine sponges Agelas dendromorpha and Cymbastella sp. We tested AgA using SW480 cells transfected with a beta-catenin dependent reporter gene expression system. Using this assay, we found that AgA is a potent Wnt signaling inhibitor with IC50 of 12nM. AgA also inhibits the expression of reporter genes in HEK293 cells cotransfected with either Wnt1 or mutated (dominant-active) beta-catenin, suggesting that its mechanism of action is independent of the beta-catenin degradation complex. Moreover, real time-PCR results show that Lef1, a classic Wnt/beta-catenin target gene highly expressed in CLL, is down-regulated in primary CLL cells incubated with AgA at nanomolar concentrations.
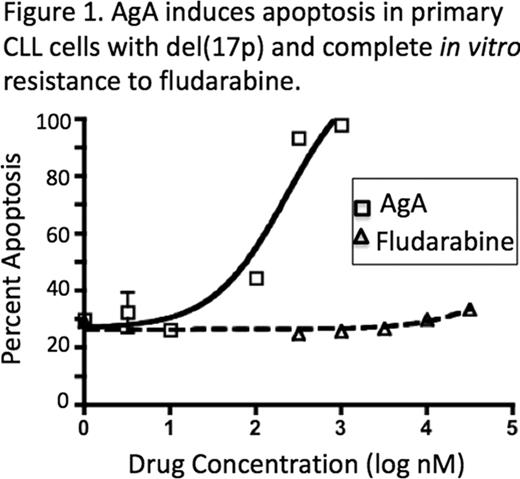
AgA, but not structurally related compounds agelastatin C and agelastatin D, selectively induces apoptosis in CLL B-cells (mean IC50 = 56nM) compared with peripheral blood mononuclear cells from healthy volunteers (mean IC50 = 250nM). Interestingly, AgA induces apoptosis at nanomolar concentrations even in samples from CLL patients who had no clinical response to fludarabine, samples with 17p deletion or TP53 gene mutations, and samples in which p53 deficiency was determined by in vitro chemoresistance and absence of inducible p21, CD95, and DR5 after irradiation (Figure 1). In addition, AgA induces apoptosis on CLL cells that are maintained in co-culture conditions with stromal cells, which typically increases leukemia cell viability. This suggests that AgA is capable of disrupting pro-survival signals derived from the microenvironment, including Wnt mediated activation.
In conclusion, AgA has potent activity against CLL cells in vitro. Our studies show that AgA inhibits Wnt/beta-catenin signaling at nanomolar concentrations, induces apoptosis in CLL cells independently of p53, and is able to disrupt pro-survival signaling derived from stromal cell support. AgA warrants further studies and clinical development for the treatment of CLL and potentially other malignancies associated with Wnt/beta-catenin signaling.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal