Abstract
Abstract 1490
The tumor suppressor p53 is frequently mutated in human cancer, including acute myeloid leukemia (AML). In AML, p53 mutations have been associated with poor risk cytogenetics (i.e. complex karyotype, −5/−7). However, the function of p53 can also be compromised by protein stabilization and/or expression. The implications of p53 protein expression have not been studied in AML.
We assessed p53 expression by high-throughput reverse phase protein array (RPPA) technology in 511 pts (719 samples). Eleven CD34+ bone marrow (BM) and 10 normal peripheral blood (PB) lymphocyte samples were used as controls. Samples were printed as 5 serial 1: 2 dilutions in duplicate using an Aushon 2470 Arrayer. Mutational status was determined by Sanger sequencing of exons 5 through 9 of the p53 gene.
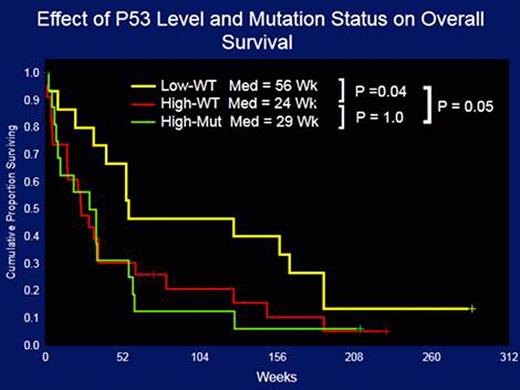
Paired PB- and BM-derived AML samples expressed similar p53 levels (p=0.25). A trend towards higher p53 expression at relapsed was observed among 47 paired diagnosis/relapse samples (p=0.07). Cases of AML-M3 and –M6 exhibited higher expression of p53 than other FAB subtypes. p53 expression directly correlated with age (p=0.01) and CD34 (p=0.001) and inversely correlated with WBC (p=0.007), BM (p=0.0001) and PB (p=0.0001) blasts, platelets (p=0.007), HLA-DR (p=0.01), CD19 (p=0.02), and survival (p=0.01). High p53 (p53high) expression level was more associated with unfavorable cytogenetics than with favorable or intermediate cytogenetics (p=0.00001). When all cytogenetic abnormalities were considered, pts with −5 had the highest levels of p53 (p=0.00001). Pts with RAS mutations, but not those with FLT3-ITD, NPM1, or IDH1/2, had lower levels of p53 protein. When pts were divided according to the level of p53 protein expression p53high was associated with lower complete remission (CR) rates (51% vs 56%; p=??) and higher relapsed rates (82% vs 62%; p=??). The median overall survival (OS) of pts with p53high and p53low were 29.8 vs. 51 wks (p=0.009). Most cases with p53high had unfavorable cytogenetics and the effect on OS was predominantly seen in that subpopulation with p53high and p53low pts living a medina of 23.4 vs. 36 wks (p=0.07), respectively. In order to determine whether the poor outcomes associated with p53high were due to the presence of a higher rate of p53 mutations among pts with p53high, we determined the p53 mutational status of 55 pts. p53high was highly correlated with the presence of p53 mutations as the latter were detected in 17/40 pts with p53high but in only 1/16 pts with p53low. Importantly, the presence of p53high, both in the presence (29 wks) or in the absence (24 wks) of p53 mutations, was associated with significantly worse overall survival compared with pts with p53low (56 wks; p=0.05, Figure 1).
Multivariate analysis indicated that p53 is a significant independent risk factor for survival in AML. The final model included: age (p=0.000001), favorable cytogenetics (0.01), unfavorable cytogenetics (p=0.00001), WBC (p=0.0005), albumin (p=0.0003), FLT3-ITD (P=0.04), and P53 (P=0.02).
p53high was positively correlated with p53pSER15 (p=0.00001), Rbp807p811 (p=0.0002), c-MET (p=0.01), FoxO3a (p=0.004), KIT (p=0.001), p38p180p182 (p0.02), BAD (p=0.0001), cleaved PARP (p=0.002), cleaved PARP (p=0.01), TCF4 (p=0.02), fibronectin (p=0.02), and hsp70 (p=0.003), and negatively with AKTp473 (p=0.01), ERK (p=0.002), mTOR (p=0.005), PI3Kp85 (p=0.002), PKCδ (p=0.00002), GAB2 (p=0.00005), beclin (p=0.007), JMJD6 (p=0.001), Gata3 (p=0.02), p21 (p=0.01), and Mdm2 (p=0.001).
Our results suggest that high levels of p53 protein constitute a powerful marker of short survival in AML. This effect is independent of p53 mutational status. The poor outcome of pts with high level of expression of p53 in the absence of p53 mutations suggests that the p53 pathway may be functionally perturbed in a much higher proportion of pts with AML than previously recognized. These data support the use of p53 protein expression levels in prognostication and in the development of targeted therapeutics.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal