Abstract
Abstract 1440
Improved Post-Induction Chemotherapy Does Not Abrogate Prognostic Significance of Minimal Residual Disease (MRD) for Children and Young Adults with High Risk Acute Lymphoblastic Leukemia (ALL). A Report from Children's Oncology Group (COG) Study AALL0232.
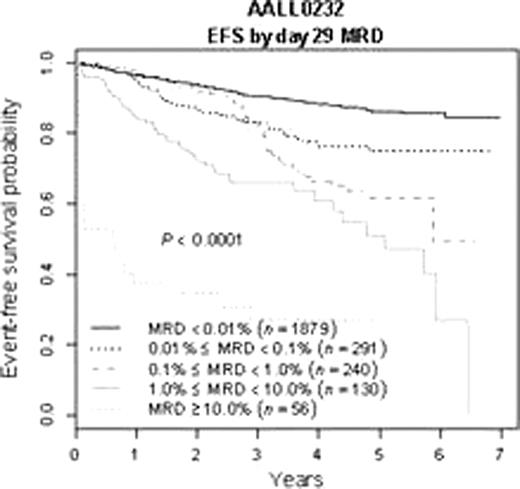
5 year EFS for end-induction MRD positive (>=0.01%) patients was 63±5% vs. 86±2% for MRD negative patients. However, patients who were MRD positive at end induction who became negative by end consolidation had improved 5y EFS of 79±9%(n=136) compared to 52±14% for those who remained MRD positive(n=52) (p=.0012). Both end induction MRD positive and negative patients benefitted from HD-MTX vs. C-MTX, though the effect was small and did not reach statistical significance for MRD positive patients. By contrast, end-induction MRD was highly predictive of outcome for patients receiving either HD-MTX or C-MTX.
5 y EFS as a function of MRD status and IM regimen.
| End induction MRD . | Capizzi . | HDMTX . | P value . |
|---|---|---|---|
| <.01% | 84 ± 3% | 88 ± 2% | .04 |
| >.01% | 59 ± 6% | 67 ± 7% | .12 |
| P value | <.0001 | <.0001 |
| End induction MRD . | Capizzi . | HDMTX . | P value . |
|---|---|---|---|
| <.01% | 84 ± 3% | 88 ± 2% | .04 |
| >.01% | 59 ± 6% | 67 ± 7% | .12 |
| P value | <.0001 | <.0001 |
End induction MRD negative patients <10y receiving DEX had better outcome than those getting PRED (5 y EFS 92±3% vs. 87±4% P=.027) while MRD positive patients or those>10y showed no difference. However, DEX patients <10y if anything had a slightly higher rate of end induction MRD positivity than those given PRED (22% vs. 17%, p=.073). In multivariate analysis, end consolidation MRD was the most powerful prognostic factor for the small subset of patients in whom this was assessed. Excluding this, end induction MRD was the most significant variable; age, white blood cell count, day 15 marrow morphology and HD-MTX vs. C-MTX were also significant. We conclude that MRD remains the most powerful prognostic factor even in the context of improved therapy. Additionally, for those patients who were MRD positive at end induction, achieving MRD negative status by end consolidation improved outcome significantly. The higher frequency of MRD in younger patients receiving DEX calls into question the validity of using end induction MRD as a surrogate for outcome when testing novel interventions during induction therapy.
Borowitz:BD Biosciences: Research Funding. Wood:BD Biosciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal