Abstract
Abstract 1425
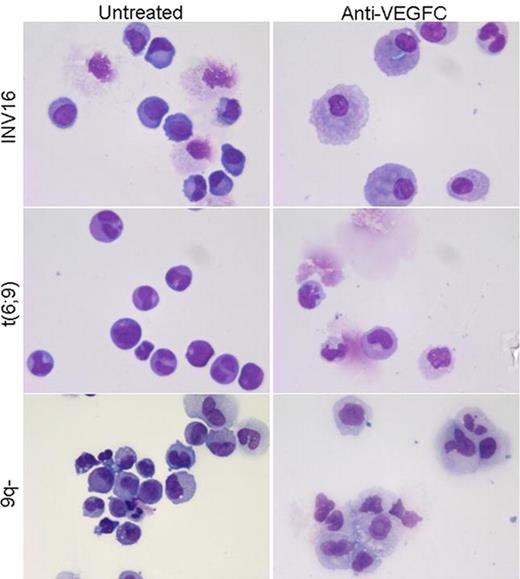
Previously, it was demonstrated that exogenous addition of vascular endothelial growth factor C (VEGFC) increased the leukemic cell viability, reduced apoptosis via activation of Bcl-2, and decreased chemotherapy induced apoptosis via its receptor FLT-4 (Further revert to as VEGFR3) (Dias et al. Blood 2002). Furthermore, it was shown that VEGFC promotes angiogenesis by induction of COX-2 through VEGFR3 activation in THP-1 cells (Chien et al. Carcinogenesis 2005). We have previously found that endogenous VEGFC expression is associated with decreased drug responsiveness in childhood acute myeloid leukemia (AML), both in vitro as well as in vivo (de Jonge et al. Clinical Cancer Research 2008). In addition, high VEGFC mRNA expression is strongly associated with reduced complete remission and overall survival in adult as well as pediatric AML (de Jonge et al. Blood 2010). It was thought that the leukemic blast population is organized as a hierarchy, whereby leukemia initiating cells (LICs) reside at the top of this hierarchy, and it is only these cells that have the capacity to engraft in non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice. The LIC is thought to be enriched in the CD34+ leukemic cell fraction and is shown to expand in vitro using a myeloid cytokine mix of IL-3, TPO, and G-CSF in colony forming cell (CFC) assays and long-term culture-initiating cell (LTC-IC) assays (Guan et al. Exp. Hematol. 2002, van Gosliga et al. Exp. Hematol. 2007). Moreover, LTC-IC assays performed in limiting dilution detect the in vitro outgrowth potential of stem-like cells that reside underneath the stromal cell layer. In this study, we set out to investigate the potential of anti-VEGFC treatment as an inhibitor of the outgrowth of LICs within the CD34+ fraction of primary AML samples. First, we determined the possibility of an autocrine loop for VEGFC in AML. Pediatric AML cell (n=7) derived VEGFC levels were found to be 1.4-fold increased (P =.008) compared to secreted VEGFC levels from normal bone marrow (NBM) cells (n=4). Pediatric AML blast cells showed KDR (further revert to as VEGFR2) membrane expression in 44 out of 50 patient samples (varying 8–99% of the total blast population), whereas on NBM cells VEGFR2 expression was below 5%. VEGFR3 expression was below 5% on both leukemic blasts and NBM cells. We evaluated the effect of anti-VEGFC (VGX-100, kindly provided by Vegenics, used at a concentration of 30 μg/ml) treatment on the CD34+ isolated compartment of pediatric AML bone marrow samples. Anti-VEGFC treatment reduced the outgrowth potential of AML derived CD34+ cells (n=2) with >25% in CFC assays. Interestingly, morphological analysis revealed a 3-fold enhanced formation of macrophages. LTC-IC assays demonstrated a (15% to 50%) decrease in the long-term growth of CD34+ isolated AML cells in 3 out of 4 patient samples. Morphological characterization of the suspension cells suggested a shift in development along the myelomonocytic lineage after two weeks of anti-VEGFC treatment. With FACS analysis, these cells showed a higher number of cells stained positive for CD11b, and CD14, and lower numbers where positive for CD34. Anti-VEGFC treated LTC-IC assays in limiting dilution demonstrated a (44% and 74%) reduction in the outgrowth potential of long-term cultured CD34+ isolated AML cells and blocked the erythroid colony formation in 2 out of 3 patient samples. Anti-VEGFC treatment did not have an effect on the outgrowth of CD34+ sorted NBM cells in the various assays (n=2). In conclusion, anti-VEGFC treatment of the CD34+ isolated fraction from primary pediatric AML samples showed a reduction of AML outgrowth. Differentiating cells are skewed to the myelomonocytic lineage upon anti-VEGFC treatment. We hypothesize that deprivation of VEGFC in primary CD34+ AML cell cultures results in enhanced leukemic cell death and abates an important proliferation signal for AML cells. Yet, further investigations are warranted. Close modal
Figure 1.
Skewing of LTC-IC assay suspension cells towards the myelomonocytic lineage upon anti-VEGFC treatment. MGG stained cytospins of suspension cells of the LTC-IC co-culture obtained during demi-depopulation at week 2.
Figure 1.
Skewing of LTC-IC assay suspension cells towards the myelomonocytic lineage upon anti-VEGFC treatment. MGG stained cytospins of suspension cells of the LTC-IC co-culture obtained during demi-depopulation at week 2.
Disclosures:
Baldwin:Circadian Technologies Limited: Employment. Robert:Circadian Technologies Limited: Employment, Membership on an entity's Board of Directors or advisory committees.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal