Abstract
Abstract 1344
Although reports are emerging of hematologic responses associated with iron chelation therapy in patients with myelodysplastic syndromes (MDS, eg, Gattermann et al. Blood 2010;116(21):abst 2912), there are limited studies in aplastic anemia (AA) patients. The EPIC study enrolled 116 transfusion-dependent AA patients with iron overload (Cappellini et al. Haematologica 2010; Lee et al. Blood 2010); deferasirox treatment resulted in a decrease in iron overload assessed by serum ferritin. As iron overload has a suppressive effect on erythroid progenitors (Hartmann et al. Blood 2008;112(11):abst 2694), it is of interest to evaluate hematologic responses with the iron chelator deferasirox in AA patients from the EPIC trial.
Full study design and inclusion/exclusion criteria for EPIC have been described (Cappellini et al. Haematologica 2010). Deferasirox dose was initiated at 20 mg/kg/day with adjustments of 5–10 mg/kg/day up to 40 mg/kg/day based on serum ferritin trends and safety assessments. For this post-hoc analysis of hematologic response and the classification of patients, UK treatment guideline criteria for AA diagnosis (2 or 3 of the following: hemoglobin [Hb] <100 g/L, platelets <50 ×109/L, neutrophils <1.5 × 109/L) and hematologic response were used (Marsh et al. Br J Haematol 2009). Bone marrow data and reticulocyte counts were not recorded in EPIC, hence patients were classified as “severe” AA based on fulfillment of platelet and neutrophil criteria. For patients with severe AA, hematologic response was defined as: no response=still severe; partial response=transfusion independent, no longer meeting criteria for severe disease; complete response=normal Hb for age and neutrophil count >1.5 × 109/L and platelet count >150 × 109/L. For patients with “non-severe” AA, hematologic response was defined as: no response=worse or not meeting criteria for partial or complete response; partial response=transfusion independence (if previously dependent), or doubling or normalization of at least one cell line, or increased Hb >3 g/dL if initially <6 g/dL, or increased neutrophils >0.5 × 109/L if initially <0.5 × 109/L, or increased platelets >20 × 109/L if initially <20 × 109/L; complete response=same criteria as severe disease. All responses were confirmed with a second measure 28 days after the first assessment. Transfusion independence was defined as at least 1 8-week period without transfusion. Serum ferritin changes from baseline to end of study (EOS) were assessed for hematologic responders and non-responders.
Of 116 iron-overloaded AA patients, 72 (62%) had evaluable hematologic parameters (according to UK criteria) at baseline and EOS; 9 (12.5%) and 63 (87.5%) were considered severe AA and non-severe AA, respectively. Thirty-five (48.6%) patients had a partial hematologic response; 33/63 [52.4%] with non-severe AA and 2/9 [22.2%] with severe AA.
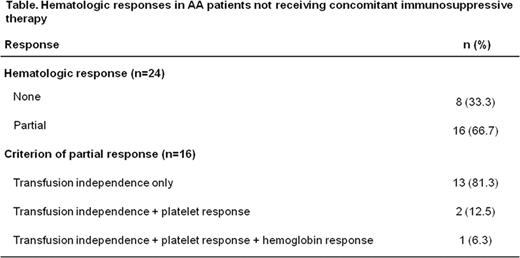
Forty-eight (67%) patients received at least one concomitant immunosuppressive treatment (IST); partial hematologic response was observed in 19/48 (39.6%) of these patients. As IST can influence hematologic response, analyses were focused on patients receiving deferasirox without IST. Twenty-four patients received deferasirox without concomitant IST; partial hematologic response was observed in 16/24 [66.7%] patients, all of whom became transfusion-independent, with 2 patients having an additional platelet response and 1 patient having an additional platelet and Hb response (Table). Median time to response was 42 days. For patients without concomitant IST, overall median change in serum ferritin from baseline to EOS was greater in partial hematologic responders (–2295 ng/mL, range –15,704 to 489 [n=16]) compared with non-responders (–815 ng/mL, range –8506 to 3671 [n=8]; P=0.22).
Similar to MDS patients, alongside a reduction in iron overload, deferasirox may improve hematologic parameters in AA patients. Sixty-seven percent (16/24) of patients receiving deferasirox without concomitant IST had a partial hematologic response and became transfusion-independent. Changes in serum ferritin were more pronounced in partial hematologic responders compared with non-responders, suggesting that decrease in iron load could play a role in inducing a hematologic response. Further analyses are required to elucidate mechanisms of hematologic improvement with deferasirox.
Lee:Novartis: Honoraria; Alexion: Honoraria. Yoon:NK Bio: Consultancy; Celgene: Consultancy. Ganser:Novartis: Honoraria, Research Funding. El-Ali:Novartis: Employment. Habr:Novartis: Employment. Roubert:Novartis: Employment. Porter, MD on behalf of the EPIC study investigators:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal