Abstract
Abstract 1329
Environmental (matrix) elasticity (Engler et al., Cell 126: 677 (2006)) and biomechanical forces (Baruch et al., Blood 114:1975 (2009)) are important differentiation signals for stem cells. In this study, we investigated how these biomechanical/biophysical signals affect megakaryocytic (Mk) differentiation, aiming to uncover signaling mechanisms behind these effects, and establish better culture environment for Mk differentiation and platelet biogenesis. CHRF cell (Fuhrken et al., Exp. Hematol. 35: 476 (2006)), a well- established Mk cell line, was used in this study. Upon PMA stimulation, CHRF cells undergo Mk-like differentiation, including endomitosis and projection of cytoplasmic extensions.
Application of shear stress (2.5 dyne/cm2; physiological range: 1.3∼4.1 dyne/cm2) to CHRF cells attached to fibronectin-coated surfaces for two hours resulted in retraction, shedding and formation of cytoplasmic extensions (proplatelet-like structures), whereas there were no morphological changes in static culture. Shear force also accelerated endomitosis as assessed by flow-cytometry based analysis of BrdU incorporation: CHRF cells exposed for two hours to shear flow had 16% more BrdU positive cells compared to cells under static conditions. The stress responsive p53 is a regulator in both cell-cycle arrest by laminar flow (Zeng et al., JBC 278: 24594 (2003)) and Mk differentiation (Fuhrken et al., JBC 283: 15589 (2008)). To determine if p53 is involved in the response of CHRF cells to shear stress, western analysis was used to show that two hours of exposure to shear (2.5 dyne/cm2) increased the ratio of K382-acetylated p53 to total p53 by more than 188% (n=3, p<0.05) while total p53 protein expression did not change significantly. These data suggest that p53 acetylation is mediating, partially at least, the response of Mk cells to biomechanical forces.
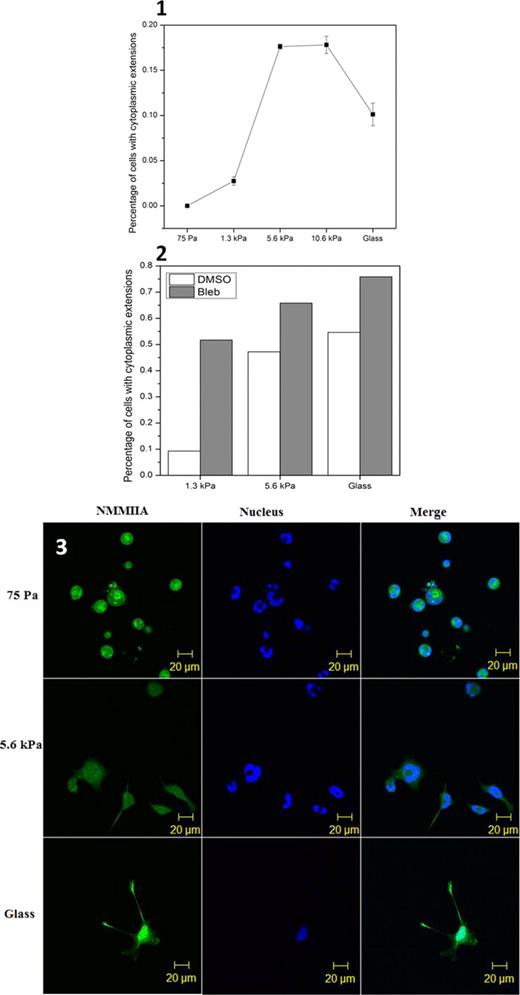
In order to examine the effect of matrix elasticity on Mk differentiation, polyacrylamide gels were used to control elasticity. We coated the gel surface with fibronectin to provide holding points for CHRF cells to sense gel elasticity. As matrix elasticity increased, the % of CHRF cells with cytoplasmic extensions increased first and decreased later (Fig. 1, n=3, Error bar: SEM). The highest percentage (17%) of CHRF cells displaying cytoplasmic extensions was observed on a gel with elasticity (ca. 8.2 kPa) mimicking the in vivo vascular niche. Non-muscle myosin IIA (NMMIIA) has been shown to serve as elasticity sensor in mesenchymal stem cells. Thus, we aimed to investigate the role of NMMIIA in the response of CHRF cells to matrix elasticity. Blebbistatin, an inhibitor of NMMIIA, minimized the differences of the percentage of cells with cytoplasmic extensions between gels with different elasticity (Fig. 2), thus suggesting that NMMIIA is involved in the response of CHRF cells to matrix elasticity and may also serve as a biological elasticity sensor. As shown by fluorescent microscopy, the cellular distribution of NMMIIA was directed by matrix elasticity (Fig. 3). On soft gels (75 Pa; n=3), NMMIIA was concentrated in the center of the cytoplasm. On a moderately stiff gel (5.6 kPa; n=3), NMMIIA was distributed across the whole cell, while on glass (the stiffest condition tested; n=3), NMMIIA moved into the nucleus and the tips of cytoplasmic extensions. Others have shown NMMIIA is involved in regulation of gene expression when it moves into the nucleus, so our observations that NMMIIA translocates into the nucleus as gel elasticity increases strongly suggests that NMMIIA may regulate gene transcription during proplatelet formation and platelet biogenesis. In future we want to investigate and prove this hypothesis.
Our data shed light on the largely unexplored role of biomechanical and biophysical parameters (of physiological significance) on Mk differentiation. The impact of these parameters is both of physiological and practical significance in the context of platelet biogenesis and ex vivo platelet generation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal