Abstract
Abstract 1285
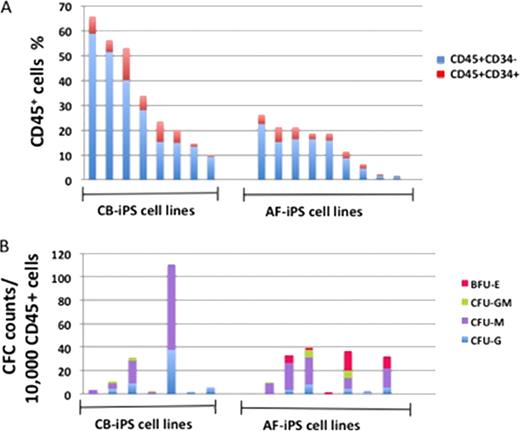
Identifying the key regulatory genes that direct human ES/iPS cell differentiation to certain lineages is required before considering these cells for their potential clinical application. It has been proposed that the cellular starting material used to generate novel iPS cell lines imparts an epigenetic memory which in turn influences the iPS lines' potential to differentiate towards certain developmental lineages. To determine novel key regulators that direct ES/iPS cell differentiation to hematopoietic lineages, we decided to compare differentiation capacity of multiple iPS cell lines generated from two neonatal mesodermal tissue derived cell types. For this, we generated multiple iPS cell lines from umbilical cord blood derived endothelial cells and amniotic fluid derived mesenchymal stem cells, using an identical dedifferentiation system/protocol. Despite the similar germ layer origin of these iPS derived cell lines, these lines showed significant differences in blood generation efficiency and lineage specification capacity. From the 8 cord blood derived iPS cell lines we tested, the average efficiency of hematopoietic (CD45+) cell generation was 34.6+/−21.2% (range 9.8 to 65.8%), of which 5.8+/− 4% were CD45+CD34+ hematopoietic progenitors. From the 9 mesenchymal stem cell derived iPS lines, the average efficiency of CD45+ hematopoietic cells was 14.2+/− 9% (range 1.6 to 26.3%), of which 2.9+/− 1.9% were CD45+CD34+ hematopoietic progenitors (Figure 1A). Note, using our standardized iPS-2-Blood differentiation protocol the efficiency of blood differentiation for individual iPS cell lines was highly reproducible. These results indicate a significant increase in the efficiency of blood generation from the endothelial derived iPS cell lines compared to the mesenchymal stem cell derived iPS cell lines, and suggest that the close ontological relationship between hematopoietic cells and endothelial cells results in the increased efficiency of blood generation seen here. We further compared the iPS cell lines for their potential to differentiate into hematopoietic cells of the myeloid, erythroid, and lymphoid lineages. Both mesoderm lineage derived iPS cell lines showed myeloid and lymphoid lineage differentiation potential as evidenced by CFU assay for presence of macrophages and granulocytes, and by 4-week OP9, and OP9-DL1 culture assays showing CD19, CD10, CD34 expression for B cells, CD1a, CD7, CD5, CD4, TCRb, CD3 expression for T cells, and CD56, CD16 for NK cells. However, further comparative analysis revealed a limited erythroid (BFU-E) potential of cord blood derived iPS cell lines compared to amniotic fluid derived iPS cell lines (Figure 1B). Most amniotic fluid derived iPS lines showed significantly erythroid cell potential, where as the cord blood derived iPS lines showed significantly reduced or no erythroid cell potential. These results suggest that the endothelial origin of cord blood derived iPS lines imparts an epigenetic memory that results in the reduced efficiency of erythroid cell generation compared with mesenchymal stem cell derived iPS lines. The difference in the efficiency of hematopoietic cell generation and lineage specification between the cord blood and amniotic fluid derived iPS lines suggests that iPS reprogramming to a pluripotent state is not complete (despite iPS cell line validation of pluripotency via teratocarcinoma assay), and significant gene expression differences exist between the lines. We are currently performing gene expression and epigenetic profile analyses of these closely related mesodermal lines, and expect that the close ontological relationship of the two mesoderm derived cell types will facilitate the deconvolution of the molecular basis of the differences. Close modal
Figure 1.
Differences in blood differentiation efficiency and lineage potential of iPS lines derived from two ontologically related cell types.
Figure 1.
Differences in blood differentiation efficiency and lineage potential of iPS lines derived from two ontologically related cell types.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal