Abstract
Abstract 1192
Recombinant factor VIIIFc (rFVIIIFc) is comprised of a B domain deleted (BDD) rFVIII protein genetically fused to the Fc domain of human immunoglobulin G1 (IgG1). Prior to secretion from HEK 293 cells, most of the rFVIIIFc is processed into a FVIII heavy chain (HC) and light chain (LC+Fc). In circulation, rFVIIIFc is complexed with von Willebrand factor (VWF) and released upon activation in a manner that is indistinguishable from native FVIII. Spontaneous dissociation of the HC and LC is thought to contribute to the loss of FVIII activity in plasma and during storage of FVIII drug products. Here we describe a single chain non-processed isoform of rFVIIIFc (SC rFVIIIFc), which may provide superior manufacturability and enhanced stability compared to native FVIII.
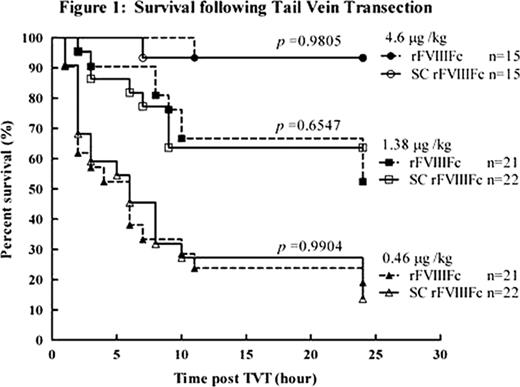
SC rFVIIIFc was purified from rFVIIIFc, which contains a fraction of the non-processed isoform. Compared to rFVIIIFc, SC rFVIIIFc showed equivalent chromogenic activity but approximately 60% reduced activity by the one stage (aPTT) assay. In a thrombin generation assay (TGA), SC rFVIIIFc also showed a reduced thrombin potential, peak thrombin and slope compared to rFVIIIFc. However, full activity of SC rFVIIIFc by aPTT was observed in the absence of VWF, suggesting release from VWF may be delayed due to covalent linkage of the a3 acidic region to the HC after Arg 1680 cleavage in SC rFVIIIFc, in contrast to a3 release and dissociation from fully processed FVIII. Delayed dissociation from VWF may explain the reduced activity observed in the aPTT assay and TGA, while full activity was observed in the two-stage chromogenic assay. A reduced rate of activation in the presence of VWF was confirmed in a modified chromogenic substrate assay with limiting thrombin as FVIII activator.
Buyue:Biogen Idec Hemophilia: Employment. Liu:Biogen Idec Hemophilia: Employment. Reidy:Biogen Idec Hemophilia: Employment. Patarroyo-White:Biogen Idec Hemophilia: Employment. Kamphaus:Biogen Idec Hemophilia: Employment. Slein:Biogen Idec Hemophilia: Employment. Pierce:Biogen Idec Hemophilia: Employment. Sommer:Biogen Idec Hemophilia: Employment. Peters:Biogen Idec Hemophilia: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal