Abstract
Abstract 1160
Thrombotic thrombocytopenic purpura (TTP) is a life-threatening generalized disorder caused by a deficiency of ADAMTS13 activity due to its gene mutations (Upshaw-Schulman syndrome), and/or acquired autoantibodies to this enzyme. ADAMTS13 specifically cleaves the peptide bond between Tyr1605 and Met1606 within the A2 domain of von Willebrand factor (VWF). Recent studies with immunoprecipitation methods using anti-VWF antibody coated beads indicated that a small portion (3–4% of the total) of plasma ADAMTS13 is bound to VWF (Feys HB et al. JTH 7:2088, 2009). This experiment determined the amount of ADAMTS13 bound to VWF in an indirect fashion, but the complex may dissociate during washing procedures or by conformation change after binding to the antibody. Thus, we used an isoelectric focusing (IEF) to separate the complex in a direct fashion. However, the molecular size of VWF-ADAMTS13 complex is assumed to be enormously huge, and therefore a regular polyacrylamide IEF gel does not properly work. So, we employed a large-pore composite IEF gel consisting 0.75% agarose and 1.25% polyacrylamide containing 2% of Pharmalyte (pI range 3.0–10). By this method followed by western blot detection using a non-neutralizing anti-ADAMTS13 monoclonal antibody (WH2-11-1), we identified that an ADAMTS13-VWF complex is detected as a sharp band at pI 7.4. The specificity of this band was identified by a lack in plasma of type 3 von Willebrand disease (VWD), and a new emergence of the band in type 3 VWD plasma spiked with purified VWF (Hori et al, 57th ISTH meeting, P-MO-479). We applied this IEF analysis to detect the complex of ADAMTS13-its autoantibodies.
ADAMTS13 activity was measured by chromogenic act-ELISA, and acquired idiopathic (ai-) TTP with severe deficiency of ADAMTS13 activity due the presence of its autoantibodies is a target in this study. VWF and ADAMTS13 were purified from normal plasma. A large-pore composite IEF gel electrophoresis was performed as previously described.
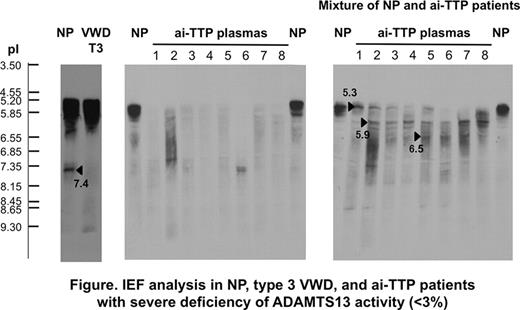
Two forms of ADAMTS13, unbound (pI 5.3) and bound (pI 7.4) to VWF in a volume of 10 uL normal plasma (NP), were directly identified on the IEF gel followed by western blotting (Fig. left). Each plasma of type 3 VWD or USS lacked the complex (VWF-ADAMTS13) band with pI 7.4, but it was generated in vitro just after spiking the purified VWF or ADAMTS13 to the respective deficient plasma by the IEF (Fig. not shown).
Next, when a volume of 3uL NP was analyzed, only one band with pI of 5.3 (5.1–5.5) was observed. In ai-TTP patients with severe deficiency of ADAMTS13 activity (<3% of the control), plasma had no or faint band of ADAMTS13 (Fig. middle). However, when we mixed the equal volume of patient plasma with ai-TTP with severe deficiency of ADAMTS13 activity and NP, the results on IEF gel showed appearance of 3 major bands with pIs of 5.3, 5.9 and 6.5, together with other many minor bands, and the unbound (free) ADAMTS13 almost disappeared (Fig. right). Then, the IgG purified from patient plasma of ai-TTP was mixed with NP or purified ADAMTS13, the complex band had a pI of 5.9 (5.5–6.3). These results indicated a possibility that the IEF analysis could be used to detect the autoantibodies to ADAMTS13, regardless of the neutralizing or non-neutralizing counterparts, in a totally different fashion to the enzyme immunoassay.
Matsumoto:Alexion Pharma: Membership on an entity's Board of Directors or advisory committees. Soejima:The Chemo-Sero-Therapeutic Research Institute: Employment. Fujimura:Baxter BioScience: Membership on an entity's Board of Directors or advisory committees; Alexion Pharma: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal