Abstract
Abstract 1094
Hypogonadotropic Hypogonadism (HH) is one of the most common morbidities in patients with transfusion-dependent anemias such as thalassemia major. Unfortunately, pituitary dysfunction is difficult to detect prior to puberty because of immaturity of the hypothalamic-pituitary-gonadal axis. We used MRI to measure pituitary R2 and volume to determine at what age these patients develop pituitary iron overload and volume loss. Additionally, we aimed to stratify the risk of clinical HH based on pituitary R2 and pituitary volume, and determine predictors of pituitary iron deposition and volume loss.
We recruited 56 patients (52 with thalassemia major and 4 with Blackfan-Diamond syndrome) to have pituitary MRIs to measure pituitary R2 and volume. Diagnosis of HH was determined by either lack of secondary pubertal characteristics by age 13 for females or age 14 for males, by primary or secondary amenorrhea in females ages 16 or older, or by the need for testosterone administration in males. Patients also had pancreas R2*, heart R2*, and liver iron concentration measured by MRI. Normative pituitary R2 and volume trends were determined by a cohort of 100 control patients.
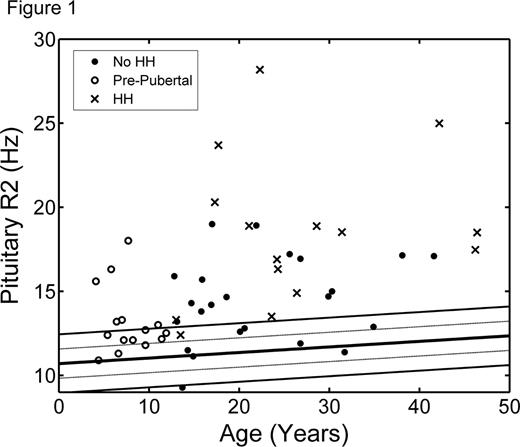
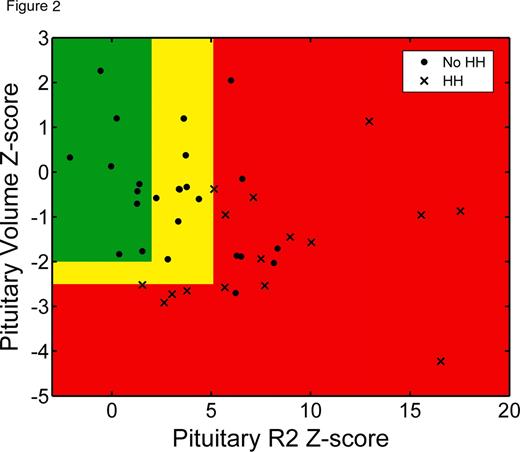
Patients were 20.4 ± 12.1 years old and well distributed by sex (31 males, 25 females). All subjects received blood transfusions every 2–4 weeks and were on appropriate chelation therapy as indicated by their physician. Mean pituitary R2 Z-score was 4.5 and mean anterior pituitary volume Z-score was −0.9. Figure 1 shows pituitary R2 values as a function of age, superimposed on normal trends from the control population. Patients with transfusional iron overload began to develop pituitary iron overload in the first decade of life; however, significant iron deposition were observed beginning in the second decade. Heavy pituitary iron deposition (Z-score > 5) and volume loss (Z-score < −2.5) were predictive of HH (Figure 2). Volume loss was highly specific (87%) but identified only half of HH cases. The remaining HH patients had heavy pituitary iron (Z-score > 5), but preserved pituitary volume. Pituitary R2 correlated significantly with serum ferritin as well as liver iron concentration, pancreatic R2*, and cardiac R2* by MRI.
Pituitary iron loading begins as early as the first decade of life in chronically transfused patients, meriting a pituitary MRI in children under 10 years old. Severe pituitary iron loading as well as volume loss throughout the second decade of life, and are predictive of HH, warranting more intensive screening in this age group. However, many patients with moderate-to-severe pituitary iron overload retained normal gland volume and function, representing a potential therapeutic window. This may also be explained by improvements in gland function observed following intensive chelation therapy. Serial tracking of pituitary iron and volume trends on age-appropriate nomograms should improve diagnostic accuracy and toxicity prophylaxis.
Wood:Novartis: Research Funding; Ferrokin Biosciences: Consultancy; Cooleys Anemia Foundation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal