Abstract
Although the role of ETS family transcriptional factor PU.1 is well established in macrophage maturation, its role in mature macrophages with reference to sepsis- related animal model has not been elucidated. Here, we report the in vivo function of PU.1 in mediating mature macrophage inflammatory phenotype by using bone marrow chimera mice with conditional PU.1 knockout. We observed that the expression of monocyte/macrophage-specific markers CD 11b, F4/80 in fetal liver cells, and bone marrow–derived macrophages were dependent on functional PU.1. Systemic inflammation as measured in terms of NF-κB reporter activity in lung, liver, and spleen tissues was significantly decreased in PU.1-deficient chimera mice compared with wild-type chimeras on lipopolysaccharide (LPS) challenge. Unlike wild-type chimera mice, LPS challenge in PU.1-deficient chimera mice resulted in decreased lung neu-trophilic inflammation and myeloperoxidase activity. Similarly, we found attenuated inflammatory gene expression (cyclooxygenase-2, inducible nitric-oxide synthase, and TLR4) and inflammatory cytokine secretion (IL-6, MCP-1, IL-1β, TNF-α, and neutrophilic chemokine keratinocyte-derived chemokine) in PU.1-deficient mice. Most importantly, this attenuated lung and systemic inflammatory phenotype was associated with survival benefit in LPS-challenged heterozygotic PU.1-deficient mice, establishing a novel protective mechanistic role for the lineage-specific transcription factor PU.1.
Introduction
Pulmonary macrophages have a prominent role in the molecular pathogenesis of the sepsis-related lung inflammatory disease, Acute Respiratory Distress Syndrome (ARDS). In ARDS patients, there is a marked population expansion and phenotypic changes of pulmonary macrophages because of the recruitment of circulating monocytes to the alveolar and interstitial space.1 Lavaged macrophages in ARDS patients display an immature monocyte-like macrophage phenotype that correlates with the severity of illness and mortality.1,2 Previously, we reported that the depletion of pulmonary macrophages by intratracheal injection of clodronated liposomes leads to reduced chemokine production, neutrophilic inflammation, and activation of nuclear factor-κB (NF-κB) in lung tissue in response to endotoxemia, demonstrating the fundamental importance of pulmonary macrophages in the pathogenesis of lung inflammation.2 The specific transcription factors and molecular mechanisms that regulate the initiation and intensity of sepsis-related lung inflammation in pulmonary macrophages are not studied in detail.
The ETS family transcriptional factor PU.1 is known to be essential for maturation of macrophages from their hematopoietic progenitors by regulating expression of genes that are involved in differentiation. When PU.1 is absent, neither M-CSF–dependent monocytic proliferation nor differentiation is observed, whereas PU.1-deficient granulocytes can undergo limited differentiation.3 The absolute amount of PU.1 gene expression regulates commitment to B-lymphocyte or macrophage differentiation. Low levels of PU.1 protein induce B-cell development, indicating that PU.1 is an important, probably critical link between innate and adaptive immunity.4 Furthermore, macrophages from the lungs of premature sheep and rats,5-7 patients with pulmonary alveolar proteinosis,8 and ethanol-fed rodents and guinea pig models are relatively deficient in PU.1.9,10 PU.1 is involved in the transcriptional regulation of GM-CSF receptor that is required for terminal differentiation of macrophages.11-13 PU.1 deficiency was correlated with alterations in innate and acquired host immunity and also regulates genes such as TLR214 and TLR415,16 that are essential in pathogen recognition. Recent studies demonstrated the genome-wide association of PU.1 with inflammatory stimuli induced gene expression patterns.17-19 Because PU.1 is required for macrophage differentiation and activation,20,21 studies on its regulatory mechanisms would be helpful in understanding the sepsis-induced inflammatory process.
In spite of the fundamental importance of PU.1 in macrophage maturation and inflammation, the biologic role of PU.1 and its influence on NF-κB activation in matured macrophages lacks direct in vivo evidence in a whole animal model. A major barrier for this study is that PU.1-deficient mice are embryonically lethal.22,23 To overcome this limitation and determine the functional significance of PU.1 in mature macrophages in sepsis-related acute inflammation, we generated bone marrow chimeras of wild-type (WT) NF-κB reporter (HLL) mice that can be made to express functionally defective PU.1. The role of functionally defective PU.1 in mature macrophages in these bone marrow chimeras was studied by single intraperitoneal injection of lipopolysaccharide (LPS). We observed that functional inactivation of PU.1 results in marked attenuation of different parameters of lung and systemic inflammation. Most importantly, the attenuation of lung injury parameters in heterozygotic PU.1-deficient mice was associated with survival benefit in a high-dose endotoxin-induced sepsis model. Our present study highlights the critical role of functional PU.1 in mature macrophages in mediating the NF-κB–dependent lung and systemic inflammation in an in vivo bone marrow chimera mouse model, suggesting that intervention in this pathway could lead to future specific therapies to protect the lungs and prevent death from multiple organ failure.
Methods
Mice
WT HLL mice express the Photinus luciferase cDNA under the control of the nuclear factor NF-κB–dependent HIV-1 long terminal repeat promoter that was developed by our laboratory group.24 Heterozygote PU/ER(T)+/− transgenic mice were generated by Dr Edward Scott (University of Florida, Gainesville). These mice carry a PU.1 allele (nuclear localization signal sequence in exon 1) fused to an estrogen receptor domain [PU/ER(T)] inducible by tamoxifen in fetal liver cells (FLCs). The mice were bred, maintained in the animal facility of University of Illinois (Chicago) and the Jesse Brown Veterans Affairs Medical Center (Chicago). All animal studies were performed in accordance with the guidelines of the Animal Care Committee of the University of Illinois at Chicago and the Jesse Brown Veterans Affairs Medical Center.
Cells
Bone marrow–derived macrophages (BMDMs) from mice under different experimental conditions were isolated according to published protocols25 and grown in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, and 20% L-cell conditioned medium.
Antibodies and fine chemicals
4-Hydroxy tamoxifen (4-OHT) for cell culture was purchased from Sigma-Aldrich, and 21-day slow release tamoxifen pellets were from Innovative Research of America (E-361). Antibodies for flow cytometry analysis were purchased from the following companies: PE rat anti–mouse CD 11b (557397) and FITC rat anti–mouse Ly-6C (553104) were from BD Biosciences Pharmingen, APC anti–mouse F4/80 (123115) was from BioLegend, mouse CD 206 Alexa Fluor was from AbD Serotec, TLR4 PE (sc-13591) was from Santa Cruz Biotechnology, and ER-MP58 (ab63860) was from Abcam. Antibodies for immunoblotting of TLR4 (sc-30002) and actin (sc-1616) proteins were purchased from Santa Cruz Biotechnology.
Southern hybridization
Genomic DNA from 14 ± 0.5-day-old fetuses was isolated and analyzed for the presence of PU/ER(T) insertion by standard protocols. The genomic DNA was digested with EcoRI, separated by electrophoresis on 1.0% agarose gels, and alkali blotted onto Biodyne A nylon membrane (77015; Pierce Chemical). Blots were probed with biotin-labeled BamHI fragment corresponding to PU.1 exon 1 and part of its 5′ flanking sequence (see Figure 1A). In some replicates of bone marrow transplantations, male PU/ER(T)+/−, PU/ER(T)+/+, or WT FLCs were transplanted in to irradiated female HLL-recipient mice. The origin of reconstituted donor macrophages in the recipient bone marrow chimera mice was confirmed by Southern hybridization with Y chromosome–specific Sry-1 probe (obtained by PCR amplification with Sry-1–specific primers forward, 5′-GAT CAG CAA GCA GCT GGG ATA CCA GTG-3′ and reverse, 5′-CTG TAG CGG TCC CGT TGC TGC GGT G-3′). The genomic sequences hybridized to biotin-labeled probe were detected by a North2South chemiluminescent-HRP substrate kit (17097; Pierce Chemical).
FLC transplantation
The details of the transplantation procedure were described in our previous study.24 In brief, FLCs were isolated from 14 ± 0.5-day-old pregnant PU/ER(T)+/− mice after heterozygote mating. PCR was used to determine the sex and genotype of the cells that were transplanted by tail vein injection into whole body–irradiated WT recipient HLL mice, and a 21-day slow-release tamoxifen pellet was implanted in the neck subcutaneous tissue to maintain a functionally active PU.1 transgene. After 21 days, a second 21-day slow-release tamoxifen pellet was placed subcutaneously to enhance reconstitution of the donor FLCs in to pulmonary macrophage population. Liposomal clodronate was prepared as described previously.26-28 Thirty days after the FLC transplantation, a single dose of liposomal clodronate was administered via intratracheal injection.26-28 After the second tamoxifen pellet was metabolized, the mice were left for an additional 1 week without tamoxifen to clear the residual circulating tamoxifen rendering the PU/ER(T) transgene inactive. LPS (10 μg/g body weight) was injected intraperitoneally in to WT, PU/ER(T)+/−, and PU/ER(T)+/+ HLL chimera mice, and different parameters indicative of systemic and lung inflammatory disease were measured maintaining appropriate controls.
In vivo bioluminescence imaging
Whole body bioluminescence imaging of PU/ER(T) chimera mice was performed by a Xenogen IVIS Spectrum in vivo imaging system (Caliper Life Sciences). Controls and LPS-treated mice were anesthetized, and the hair from neck-to-abdomen region was removed. These mice were injected with luciferin (1 mg/mL), and then in vivo bioluminescence was measured exactly after 10 minutes of luciferin injection. The measurements were repeated after 16 hours of LPS injection, and NF-κB activity was expressed as photon values per second per square centimeter of chest area of each mouse.
FACS analysis
Single-cell suspensions of FLCs or BMDMs (1 × 104/sample) were washed and incubated on ice for 30 minutes with appropriate fluorescently labeled antibodies. Cells were analyzed on an FACS Vantage flow cytometer (BD Biosciences) where gating was based on respective unstained cell population and isotype matching control antibodies. The data were analyzed with CellQuest software (BD Biosciences).
Bronchoalveolar lavage differential cell count
Bronchoalveolar lavage (BAL) from different groups of PU/ER(T) chimera mice was analyzed by cytospin preparations stained by HEMA 3 (Fisher Scientific) according to product instructions. The number of macrophages and neutrophils in different treatments were quantitated and compared for statistical significance.
Luciferase assay
Lung, liver, and spleen were collected from euthanized mice and frozen in liquid nitrogen immediately. Tissue homogenates were prepared in 1 × luciferase assay lysis buffer (E4030 Luciferase Assay system; Promega) and placed on ice for 20 minutes with frequent vortexing. After centrifugation, the protein concentration was determined, and to 1 mg of tissue extract from different samples, we added 100 μL of luciferase and then luciferase activity was measured using a luminometer.
MPO assay
A portion of frozen lung (250-300 mg) was weighed and homogenized in 1 mL of lysis buffer containing 50mM potassium phosphate, pH 6.0, with 5mM EDTA and 50mM hexadecyl trimethyl ammonium bromide.29 The homogenate was sonicated, freeze thawed, and centrifuged at 9500g for 15 minutes. The supernatant was mixed with assay buffer (1:30, vol/vol) and placed in a spectrophotometer for recording change in absorbance at 460 nm for 2 minutes. The assay buffer consisted of 100mM potassium phosphate, pH 6.0, H2O2 (30% stock diluted 1:100; Sigma-Aldrich), and 1 mg/mL O-dianisidine hydrochloride (D9154; Sigma-Aldrich). The results were expressed as units of myeloperoxidase (MPO) activity where 1 unit was defined as change in optical density at 460 nm per milligram of protein per minute.
RT-PCR and Northern blot analysis
RNA was extracted from cells by using an RNeasy Mini kit (74104; QIAGEN). cDNA synthesis and quantitative (q)RT-PCR was performed using standard protocols and murine TLR4-specific primers (forward, 5′-GAAGGAGTGCCCCGCTTTCACC-3′; reverse, 5′-TGGCAGCAATGGCTACACCAGGA-3′) and SYBR Green Master Mix from SA Bioscience. RT-PCR amplification was performed on a 7900HT Sequence Detection system (Applied Biosystems), and the data were collected and analyzed. Total RNA was electrophoresed and transferred on to Biodyne A nylon membrane (77015; Pierce Chemical). The resulting RNA blots were hybridized with biotin-labeled 70-bp cDNA probe from the third exon of TLR4, amplified using the same primers used for RT-PCR analysis. Similarly, equal amount of RNA was transferred on to Biodyne A membrane and probed with biotin-labeled β-actin cDNA probe.
Western blot analysis
BMDMs from different mice groups were lysed in PBS containing 1% nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and 1 × protease inhibitor cocktail (Roche Diagnostics) and sonicated briefly. Cell lysates containing equal amount of protein were electrophoresed and immunoblotted using appropriate antibodies.
Lung capillary leakage measurement
Evans Blue–labeled albumin (EBA) was administered retro-orbitally in PBS and LPS-treated mice. After 30 minutes of EBA injection, the mice were euthanized to assess vascular leakage, as described previously.30 In brief, blood was collected into heparinized tubes, and serum was separated by centrifugation at 1300g for 10 minutes. Lung homogenates were prepared to quantitate the EBA leakage. Both the lung homogenate and serum were incubated with 2 volumes of formamide for 18 hours at 60°C, centrifuged at 5000g for 30 minutes, and then the optical density of the supernatant was read at 620 and 740 nm. EBA extravasation is calculated as ratio of optical density between lung and serum formamide extracts.
Lung wet/dry ratio
The left lung from the same mice used for EBA injection was excised, weighed, and dried in the oven at 60°C for 18 hours. The difference between the lung wet/dry ratios is quantitated in controls and LPS-treated mice.31
Statistical analysis
All the experiments were repeated 3 times for consistent results, and representative data sets for flow cytometry and bioluminescence measurements are presented. For all the data sets, statistical analyses were performed with InStat (GraphPad Software) using a nonpaired t test or ANOVA, and P values were calculated.
Results
Genetic characterization of the PU/ER(T)+/− mice
PU/ER(T)+/− mice are knockin mice where the gene for full-length PU.1 is fused to the modified estrogen receptor ligand binding domain-G525R that is tamoxifen responsive. The coding sequence for the PU/ER(T) transgene is knocked into the WT PU.1 loci (exon 1 harboring the nuclear localization signal sequence) as illustrated in Figure 1A. In the presence of tamoxifen, the PU/ER(T) fusion protein is translocated into nucleus and becomes functionally active similar to WT PU.1, but in the absence of tamoxifen the fusion molecule remains in the cytoplasm and is transcriptionally inactive. The WT PU.1 gene is functionally knocked out completely by 2 alleles of the PU/ER(T) transgene insertion in homozygotes and was designated as PU/ER(T)+/+ [PU/ER(T)+/+ are not WT mice]. In heterozygotes, the single allele of PU/ER(T) was inserted in the PU.1 locus and designated as PU/ER(T)+/−. WT mice that contain normal PU.1 alleles are referred as WT throughout the study. Because PU/ER(T)+/+ homozygous embryo dies at day 18 in utero, only PU/ER(T)+/− heterozygous mice were breed to maintain PU/ER(T) gene allele. The PU/ER(T) gene allele in the transgenic mice was confirmed by Southern blot using the 484-bp probe that encompasses exon 1 and part of its 5′ sequence from the PU.1 gene (Figure 1A). EcoRI was used to digest genomic DNA, and as expected, the designated probe hybridized with genomic DNA harboring the 8.3-kb fragment of PU.1 WT allele, the 5.6-kb fragment of PU/ER(T)+/+ homozygotic allele (knockin), or both the 8.3- and 5.6-kb fragments in heterozygotic alleles (Figure 1B). To confirm homozygotic PU/ER(T) FLC phenotype, we used FACS to identify the CD 11b surface antigen that depends on functional PU.1 expression. The loss of functional PU.1 in PU/ER(T)+/+ mice results in a near abrogation of CD 11b expression in FLCs, whereas partial loss of PU.1 in PU/ER(T)+/− results in partial decrease of its expression (Figure 1C). Thus, the results of Southern blot and FACS confirmed the PU/ER(T) knockin construction strategy in terms of genotype and phenotype of FLCs that were used for transplantation in next step.
Genotypic and phenotypic characterization of PU/ER(T) mice. (A) Schematic illustration of the PU/ER(T) targeting vector map that includes the vector, the PU.1 locus, and the targeted PU.1 mutant allele generated by homologous recombination. The coding sequence for the PU/ER(T) transgene is knocked into the WT PU.1 loci (exon 1 harboring the nuclear localization signal sequence) as illustrated. Double-headed arrow corresponds to the sequence used as a probe for Southern hybridization that encompasses a part of exon 1 its 5′ sequence. The restriction sites are indicated as E1, EcoRI; H3, HindIII. (B) Southern blot analysis of WT, homozygote [PU/ER(T)+/+], and heterozygote [PU/ER(T)+/−] fetal liver genomic DNA that designates the location of the 8.3-kb WT, 8.3- and 5.60-kb PU/ER(T)+/−, and 5.6-kb PU/ER(T)+/+ bands. (C) Flow cytometry analysis of day 14 ± 1 FLC suspension from PU/ER(T)+/− mice. Numbers in the bottom right quadrates indicate percentage of cells carrying the CD 11b surface antigen. Data are representative of 3 individual measurements.
Genotypic and phenotypic characterization of PU/ER(T) mice. (A) Schematic illustration of the PU/ER(T) targeting vector map that includes the vector, the PU.1 locus, and the targeted PU.1 mutant allele generated by homologous recombination. The coding sequence for the PU/ER(T) transgene is knocked into the WT PU.1 loci (exon 1 harboring the nuclear localization signal sequence) as illustrated. Double-headed arrow corresponds to the sequence used as a probe for Southern hybridization that encompasses a part of exon 1 its 5′ sequence. The restriction sites are indicated as E1, EcoRI; H3, HindIII. (B) Southern blot analysis of WT, homozygote [PU/ER(T)+/+], and heterozygote [PU/ER(T)+/−] fetal liver genomic DNA that designates the location of the 8.3-kb WT, 8.3- and 5.60-kb PU/ER(T)+/−, and 5.6-kb PU/ER(T)+/+ bands. (C) Flow cytometry analysis of day 14 ± 1 FLC suspension from PU/ER(T)+/− mice. Numbers in the bottom right quadrates indicate percentage of cells carrying the CD 11b surface antigen. Data are representative of 3 individual measurements.
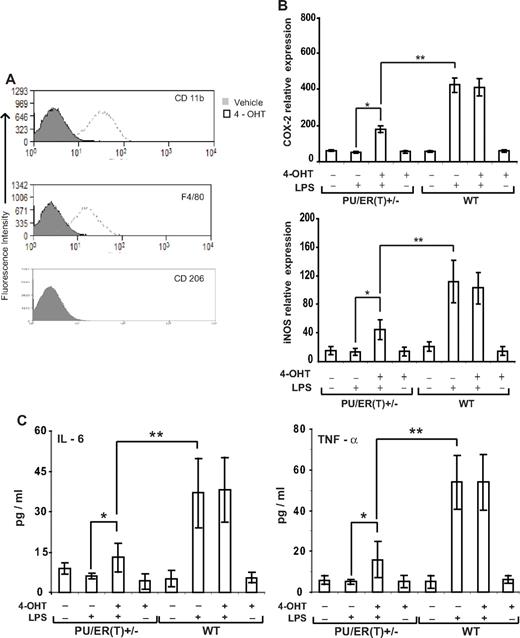
Inflammatory phenotype of BMDMs from PU/ER(T)+/− mice
BMDMs from PU/ER(T)+/− heterozygotes were isolated and characterized for their phenotype in the presence and absence of 4-OHT and endotoxin responsiveness. PU/ER(T)+/− BMDM cells were treated with 100nM 4-OHT for 24 hours and analyzed by flow cytometry for expression of cell surface CD 11b, F4/80, and CD 206. Tamoxifen treatment significantly increased the population of cells expressing CD 11b and F4/80 but had no effect on CD 206–expressing cells (Figure 2A). To analyze the inflammatory gene expression, the PU/ER(T)+/− BMDMs were treated with 4-OHT for 24 hours and then challenged with LPS at 100 ng/mL for 16 hours before collecting the cells and culture supernatant for analysis. The levels of cyclooxygenase (COX)–2, inducible nitric-oxide synthase (iNOS), and arginase 1 mRNA were measured using qRT-PCR. Compared with ethanol-treated cells, LPS challenge pronouncedly increased COX-2 and iNOS mRNA but not arginase 1 expression in 4-OHT–pretreated cells (Figure 2B, data not shown for arginase 1). The culture supernatant was analyzed by multiplex ELISA to quantitate the release of IL-6, TNF-α, IL-10, and 2 subunits of IL-12 (p40, p70). The secretion of IL-6 and TNF-α in response to LPS increased in cells pretreated with 4-OHT but not in ethanol-treated controls (Figure 2C). However, there was no effect of 4-OHT–inducible PU.1 on secretion of IL-10 and 2 subunits of IL-12 (data not shown). Results from all these experiments clearly indicate that the PU/ER(T) macrophage surface CD 11b, F4/80 expression, and inflammatory gene expression are dependent on functional PU.1. Therefore, we used PU/ER(T)+/− heterozygotes and PU/ER(T)+/+ bone marrow chimera mice to further study the role of PU.1 deficiency on mature macrophage function in sepsis-induced lung injury.
Immunophenotype of PU/ER(T)+/− BMDMs. (A) BMDMs from PU/ER(T)+/− mice were treated with 4-OHT for 24 hours, and then the expression of CD 11b, F4/80, and CD 206 was measured by FACS analysis. Data are representative of 3 individual measurements. (B) PU/ER(T)+/− BMDM cells were treated with and without 4-OHT for 24 hours and challenged with 100 ng/mL LPS. Total cellular RNA was isolated and reverse transcribed. Relative expression levels of COX-2 and iNOS were analyzed by qRT-PCR. Data are representative of the average of 3 individual measurements. (C) PU/ER(T)+/− BMDMs were treated with and without 4-OHT and challenged with 100 ng/mL LPS. The culture supernatant was analyzed for IL-6 and TNF-α by multiplex ELISA. Data represent mean ± SEM of 4 individual measurements (*P < .01 compared with ethanol-treated cells, **P < .001 compared with PU/ER(T)+/− LPS treatment).
Immunophenotype of PU/ER(T)+/− BMDMs. (A) BMDMs from PU/ER(T)+/− mice were treated with 4-OHT for 24 hours, and then the expression of CD 11b, F4/80, and CD 206 was measured by FACS analysis. Data are representative of 3 individual measurements. (B) PU/ER(T)+/− BMDM cells were treated with and without 4-OHT for 24 hours and challenged with 100 ng/mL LPS. Total cellular RNA was isolated and reverse transcribed. Relative expression levels of COX-2 and iNOS were analyzed by qRT-PCR. Data are representative of the average of 3 individual measurements. (C) PU/ER(T)+/− BMDMs were treated with and without 4-OHT and challenged with 100 ng/mL LPS. The culture supernatant was analyzed for IL-6 and TNF-α by multiplex ELISA. Data represent mean ± SEM of 4 individual measurements (*P < .01 compared with ethanol-treated cells, **P < .001 compared with PU/ER(T)+/− LPS treatment).
Immunophenotype of PU/ER(T)+/− heterozygotes depends on functional PU.1
PU/ER(T)+/− mice are resistant to LPS-induced lung injury and septic shock.
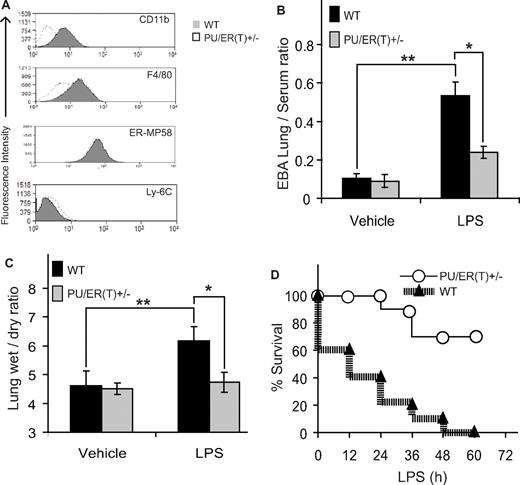
Because the PU/ER(T)+/− BMDMs distinctly showed a PU.1-dependent inflammatory phenotype, we studied the PU.1-dependent lung and systemic inflammation in PU/ER(T)+/− heterozygotes. First, to determine the expression of macrophage surface markers, we compared the expression of CD 11b, F4/80, ER-MP58, and Ly-6C antigen on the surface of BMDMs from PU/ER(T)+/− and WT littermates (Figure 3A). As observed in FLCs (Figure 1C), compared with WT mice the PU/ER(T)+/− mice had fewer CD 11b-positive BMDMs, which correlates with the presence of single copy of functional PU.1 (Figure 3A). Interestingly, the expression of immature macrophage markers ER-MP58 and Ly-6C were not significantly different in BMDMs isolated from WT and PU/ER(T)+/− mice (Figure 3A). To assess the effect of deletion of functional PU.1 on LPS-induced septic shock, the PU/ER(T)+/− and WT mice were injected intraperitoneally with 10 mg/kg LPS to determine lung injury in terms of lung albumin capillary leakage and wet/dry ratio. The lung albumin leakage and wet/dry ratio increased significantly in WT mice compared with PU/ER(T)+/− mice treated with LPS (Figure 3B-C). To assess the LPS-induced septic shock in WT and PU/ER(T)+/− mice, a high dose of LPS (40 mg/kg body weight) was injected intraperitoneally, and survival of the animals was monitored. PU/ER(T)+/− mice were remarkably resistant to lethal effect of LPS (Figure 3D). WT animals showed 80% mortality at 60 hours and reached 100% mortality within 48 hours, whereas PU/ER(T)+/− mice showed only 30% mortality up to 60 hours. Although surviving PU/ER(T)+/− mice showed mild signs of LPS-induced shock during the initial few hours, they completely recovered after 72 hours, suggesting a significant protective effect of PU.1 deletion against LPS-induced septic shock (Figure 3D). Thus, the immunologic phenotype of the PU/ER(T)+/− heterozygotes suggests that single PU.1 allele knockdown is sufficient for the in vivo protective effect against LPS-induced septic shock or lung injury. The observed results were further confirmed using allogenic PU.1 bone marrow chimera mice that allowed us to asses the effect of conditional deletion of both alleles of PU.1 gene in mature macrophages during endotoxin-induced inflammation.
Immunophenotype of PU/ER(T)+/− heterozygote mice. (A) BMDMs from heterozygote PU/ER(T)+/− and WT mice were subjected to flow cytometry analysis for the presence of CD 11b, F4/80, ER-MP58, and Ly-6C antigens. (B) EBA (2%) was injected retro-orbitally in to anesthetized PBS and LPS-treated WT and PU/ER(T)+/− mice. After 30 minutes, mice were euthanized, and lung vascular permeability was determined by quantifying EBA extravasation and (C) left lung wet/dry weight ratio. Data represent mean ± SEM of 4 individual measurements (*P < .01 compared with WT LPS treatment, **P < .001 compared with PBS-injected controls). (D) Survival of PU/ER(T)+/− and WT mice after intraperitoneal injection of LPS (40 mg/kg) was monitored at regular time intervals up to 72 hours (n = 10).
Immunophenotype of PU/ER(T)+/− heterozygote mice. (A) BMDMs from heterozygote PU/ER(T)+/− and WT mice were subjected to flow cytometry analysis for the presence of CD 11b, F4/80, ER-MP58, and Ly-6C antigens. (B) EBA (2%) was injected retro-orbitally in to anesthetized PBS and LPS-treated WT and PU/ER(T)+/− mice. After 30 minutes, mice were euthanized, and lung vascular permeability was determined by quantifying EBA extravasation and (C) left lung wet/dry weight ratio. Data represent mean ± SEM of 4 individual measurements (*P < .01 compared with WT LPS treatment, **P < .001 compared with PBS-injected controls). (D) Survival of PU/ER(T)+/− and WT mice after intraperitoneal injection of LPS (40 mg/kg) was monitored at regular time intervals up to 72 hours (n = 10).
Generation of PU/ER(T) chimeric mice by bone marrow transplantation.
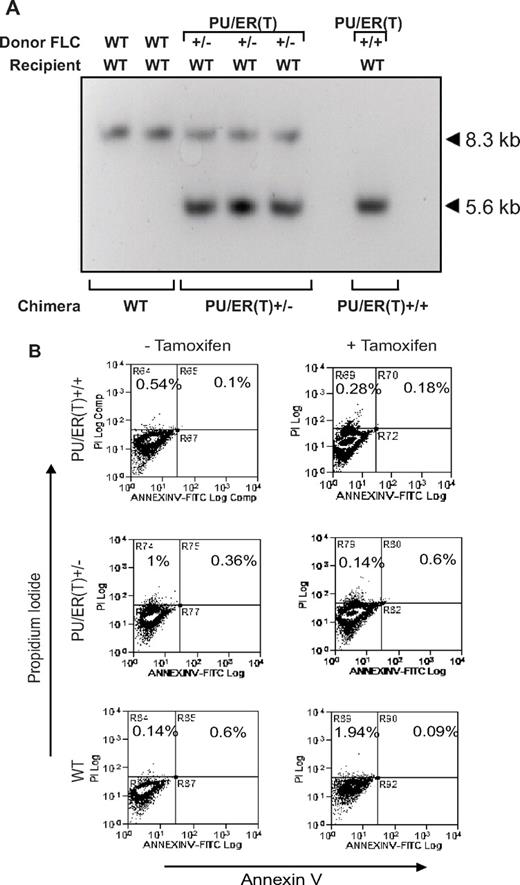
After determining sex and PU.1 genetic background by PCR, the FLCs were used as source of donor precursors of hematopoietic cells, to generate bone marrow chimera mice as described in “Methods” (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and our previous study.26-28 Bone marrow reconstitution by donor FLCs in recipient chimera mice was confirmed by Southern hybridization using biotin-labeled BamHI fragment as probe (Figure 4A). Only the bands corresponding to WT or the heterozygote or homozygote genotype (same as the donor FLCs) were detected from the WT, heterozygote, or homozygote bone marrow chimera mice. These results indicate that fetal liver transplantation could segregate the WT genotype from the PU/ER(T) genotype, because the Southern hybridization signal corresponding to WT genotype was not detected in either the heterozygote or homozygote bone marrow chimera mice. The bone marrow reconstitution in female HLL chimera mice also was confirmed by Southern hybridization using biotin-labeled Y chromosome specific Sry-1 probe where donor FLCs were transplanted from male PU/ER(T) mice. As shown in supplemental Figure 2, the alveolar macrophage genomic DNA show positive signal for Sry-1 (3.5-kb band) characteristic of Y chromosome in female HLL recipients, indicating successful engraftment of donor cells.
Reconstitution of donor FLC in to mature macrophages in bone marrow chimera mice. (A) Sixty days after FLC transplantation, genomic DNA from alveolar macrophages from resulting chimera mice was subjected to Southern hybridization as described in “Methods.” The hybridization signals in WT (8.3 kb), PU/ER(T)+/− (8.3 and 5.6 kb), and PU/ER(T)+/+ (5.6 kb) indicated the genotype of the resulting chimera mice. (B) Alveolar macrophages from the WT, PU/ER(T)+/−, and PU/ER(T)+/+ bone marrow chimera mice were collected before and after clearance (45th and 55th day) of circulating tamoxifen. Cells were analyzed by FACS for Annexin V/F4/80 and propidium iodide (PI) binding to assess cell apoptosis.
Reconstitution of donor FLC in to mature macrophages in bone marrow chimera mice. (A) Sixty days after FLC transplantation, genomic DNA from alveolar macrophages from resulting chimera mice was subjected to Southern hybridization as described in “Methods.” The hybridization signals in WT (8.3 kb), PU/ER(T)+/− (8.3 and 5.6 kb), and PU/ER(T)+/+ (5.6 kb) indicated the genotype of the resulting chimera mice. (B) Alveolar macrophages from the WT, PU/ER(T)+/−, and PU/ER(T)+/+ bone marrow chimera mice were collected before and after clearance (45th and 55th day) of circulating tamoxifen. Cells were analyzed by FACS for Annexin V/F4/80 and propidium iodide (PI) binding to assess cell apoptosis.
To assess the viability of macrophages without functional PU.1 (when circulating tamoxifen is cleared), we measured the population of apoptotic alveolar macrophages (only F4/80-positive cells) by annexin V/propidium iodide staining. Alveolar macrophages in WT, PU/ER(T)+/−, and PU/ER(T)+/+ bone marrow chimera mice groups showed no difference in apoptotic macrophage numbers (Figure 4B). This result indicates that the differential LPS response among WT and PU.1-deficient mice groups in subsequent experiments was not because of difference in viable population of pulmonary macrophages.
Immunophenotype of PU/ER(T) bone marrow chimera mice depends on functional PU.1
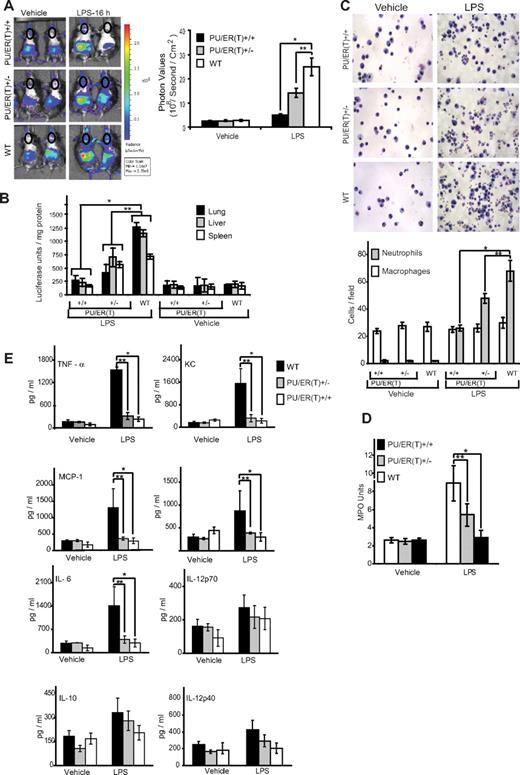
As described in “Methods,” FLCs of WT, PU/ER(T)+/−, and PU/ER(T)+/+ genetic background were transplanted into whole body–irradiated WT HLL mice and subjected to tamoxifen selection pressure. HLL mice contain NF-κB–driven luciferase reporter gene that can be measured by bioluminescence in live animals as a surrogate marker of NF-κB activation in various organs and tissues.24 Furthermore, to help maximal reconstitution of pulmonary macrophages with donor FLCs, the mice were treated with clodronated liposomes to eliminate the resident recipient pulmonary macrophages as described in “Methods.” On the 55th day after FLC transplantation, which is 7 days after the second 21-day tamoxifen pellet was presumed to have been consumed and eliminated (according to manufacturer's specification), the mice were treated with a single intraperitoneal dose (10 mg/kg body weight) of endotoxin, and whole body bioluminescence was measured at 16 hours, as described in “Methods.” There was marked reduction in the levels of bioluminescence that is an indirect measure of NF-κB activity, in the lungs of HLL-PU/ER(T)+/+ and HLL-PU/ER(T)+/− chimeras (Figure 5A circled chest area) compared with WT chimera mice in response to LPS treatment (Figure 5A). Similarly, the luciferase activity measured in postmortem lung, liver, and spleen tissue was markedly attenuated in the HLL-PU/ER(T)+/+ and HLL-PU/ER(T)+/− bone marrow chimera mice compared with WT chimera. Together, these observations suggest that PU.1 is essential for mediating LPS-induced NF-κB activation during lung and systemic inflammation.
Immunophenotype of WT, PU/ER(T)+/−, and PU/ER(T)+/+ bone marrow chimera mice. (A) WT, PU/ER(T)+/−, and PU/ER(T)+/+ bone marrow chimera mice were injected with luciferin 1 mg/mL and imaged to measure bioluminescence which is an indirect indicator of NF-κB activation at 0 and 16 hours after LPS (10 mg/kg body weight) injection. NF-κB activation in terms of chest photon values per second per square centimeter area was quantitated (represented in circled chest area). Data represent mean ± SEM of 5 individual measurements for LPS treatment and controls (*P < .001 compared with WT LPS treatment, **P < .01 compared with WT LPS treatment). (B) Lung, liver, and spleen tissue from WT, PU/ER(T)+/−, and PU/ER(T)+/+ bone marrow chimera mice were collected, and homogenates were prepared in luciferase assay tissue lysis buffer (Promega). The luciferase activity was measured in different tissue homogenates using luciferin as the substrate. Data represent mean ± SD of 5 individual measurements for LPS and vehicle treatments (*P < .001 compared with WT LPS treatment, **P < .01 compared with WT LPS treatment). (C) Sixteen hours after LPS treatment, BAL fluid was collected from different groups of mice; cytospin slides were prepared and stained with HEMA 3. The numbers of macrophages and neutrophils per field view of microscope were quantitated. Data represent mean ± SD of 3 individual measurements from different animals (*P < .001 compared with WT LPS treatment, **P < .01 compared with WT LPS treatment). (D) Lung tissue homogenates from different groups of bone marrow chimera mice were prepared and analyzed for MPO activity. One unit of MPO activity was defined as change in absorbance at 460 nm per milligram of protein per minute. Data represent mean ± SD of 3 individual measurements from different animals (*P < .001 compared with WT LPS treatment, **P < .01 compared with WT LPS treatment). (E) Blood serum was collected from vehicle and LPS-treated bone marrow chimera mice, and levels of IL-6, MCP-1, KC, TNF-α, IL-1β, IL-10, IL-12p40, and IL-12p70 were measured by multiplex ELISA. Data represent mean ± SD of 4 individual measurements from different animals (*P < .001 compared with WT LPS treatment, **P < .01 compared with WT LPS treatment).
Immunophenotype of WT, PU/ER(T)+/−, and PU/ER(T)+/+ bone marrow chimera mice. (A) WT, PU/ER(T)+/−, and PU/ER(T)+/+ bone marrow chimera mice were injected with luciferin 1 mg/mL and imaged to measure bioluminescence which is an indirect indicator of NF-κB activation at 0 and 16 hours after LPS (10 mg/kg body weight) injection. NF-κB activation in terms of chest photon values per second per square centimeter area was quantitated (represented in circled chest area). Data represent mean ± SEM of 5 individual measurements for LPS treatment and controls (*P < .001 compared with WT LPS treatment, **P < .01 compared with WT LPS treatment). (B) Lung, liver, and spleen tissue from WT, PU/ER(T)+/−, and PU/ER(T)+/+ bone marrow chimera mice were collected, and homogenates were prepared in luciferase assay tissue lysis buffer (Promega). The luciferase activity was measured in different tissue homogenates using luciferin as the substrate. Data represent mean ± SD of 5 individual measurements for LPS and vehicle treatments (*P < .001 compared with WT LPS treatment, **P < .01 compared with WT LPS treatment). (C) Sixteen hours after LPS treatment, BAL fluid was collected from different groups of mice; cytospin slides were prepared and stained with HEMA 3. The numbers of macrophages and neutrophils per field view of microscope were quantitated. Data represent mean ± SD of 3 individual measurements from different animals (*P < .001 compared with WT LPS treatment, **P < .01 compared with WT LPS treatment). (D) Lung tissue homogenates from different groups of bone marrow chimera mice were prepared and analyzed for MPO activity. One unit of MPO activity was defined as change in absorbance at 460 nm per milligram of protein per minute. Data represent mean ± SD of 3 individual measurements from different animals (*P < .001 compared with WT LPS treatment, **P < .01 compared with WT LPS treatment). (E) Blood serum was collected from vehicle and LPS-treated bone marrow chimera mice, and levels of IL-6, MCP-1, KC, TNF-α, IL-1β, IL-10, IL-12p40, and IL-12p70 were measured by multiplex ELISA. Data represent mean ± SD of 4 individual measurements from different animals (*P < .001 compared with WT LPS treatment, **P < .01 compared with WT LPS treatment).
LPS-induced neutrophil recruitment in PU/ER(T) chimeric mice depends on functional PU.1
The differential cell counts in bronchoalveolar lavage fluid from different groups of PU/ER(T) chimera mice were compared. Interestingly, the alveolar macrophage numbers were similar in all 3 groups of bone marrow chimera mice. But, the number of neutrophils increased significantly in HLL-WT chimera mice in response to LPS stimulation. Furthermore, neutrophilic infiltration was markedly reduced in HLL-PU/ER(T)+/+ and to a lesser degree in HLL-PU/ER(T)+/− chimera mice (Figure 5C). In HLL-WT chimera mice, the increase in neutrophilic infiltration was associated with an increase in myeloperoxidase activity in lung tissue in response to endotoxin treatment (Figure 5D). The MPO activity in HLL-PU/ER(T)+/+ mice was completely attenuated and was similar to that of control mice. But, in HLL-PU/ER(T)+/− chimera mice the reduction in MPO activity was ∼ 45% to 50% of the HLL-WT mice MPO levels in response to LPS challenge (Figure 5D).
LPS induces various inflammatory cytokines in vivo leading to lung and systemic infection that result in sepsis.32,33 To determine the effect of functional inactivation of PU.1 on LPS-induced cytokine and chemokine production, we measured the levels of different cytokines such as IL-1β, IL-6, IL-12p40, IL-12p70, keratinocyte-derived chemokine (KC), MCP-1, TNF-α, and IL-10 in blood serum of WT, PU/ER(T)+/−, and PU/ER(T)+/+ chimera mice (Figure 5E). The WT chimera mice treated with LPS showed severalfold induction of serum inflammatory cytokines such as IL-1β, IL-6, KC, MCP-1, and TNF-α. As observed in PU/ER(T)+/− BMDM cells, the in vivo secretion of inflammatory cytokines in LPS-treated homozygotic and heterozygotic PU/ER(T) chimera mice was attenuated significantly compared with WT chimera mice. However, there was no significant increase in levels of IL-10 and IL-12p70 in all the 3 groups of mice.
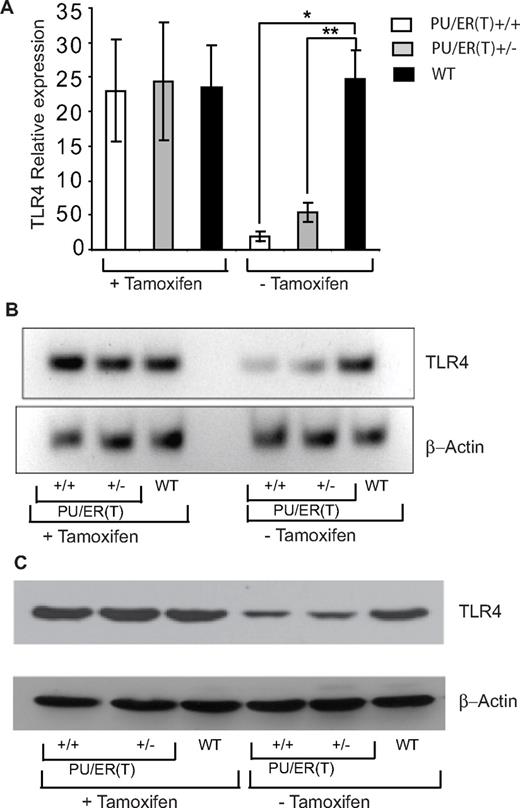
Because it has been reported that PU.1 controls the TLR4 transcription start site positioning and also its expression levels in murine macrophages,15,16 we measured the levels of TLR4 in alveolar macrophages of WT and PU/ER(T) bone marrow chimera mice. The TLR4 mRNA levels decreased in HLL-PU/ER(T)+/+ and HLL-PU/ER(T)+/− chimera mice compared with WT chimeras as measured by qRT-PCR and Northern blotting (Figure 6A-B). Alveolar macrophages from HLL-PU/ER(T)+/+ and HLL-PU/ER(T)+/− chimera that were not subjected to tamoxifen depletion showed similar levels of TLR4 mRNA expression as that of WT macrophages (Figure 6A-B). Depletion of tamoxifen also decreased the TLR4 protein levels in BMDMs of HLL-PU/ER(T)+/+ and HLL-PU/ER(T)+/− chimera mice compared with WT chimera mice (Figure 6C). In contrast, the TLR4 protein levels were of the same magnitude in HLL-PU/ER(T)+/+, HLL-PU/ER(T)+/−, and WT chimera mice that were not depleted of circulating tamoxifen (Figure 6C).
Effect of PU.1 deficiency on TLR4 expression in WT, PU/ER(T)+/−, and PU/ER(T)+/+ bone marrow chimera mice. (A) BAL macrophages were collected from WT, PU/ER(T)+/−, and PU/ER(T)+/+ bone marrow chimera mice on the 45th and 55th day (before and after clearance of circulating tamoxifen), and then total RNA was isolated. Expression levels of TLR4 were measured by qRT-PCR. Data are representative of the average of measurements from 4 different mice. (B) Total RNA isolated as described in panel A was electrophoresed and transferred on to Biodyne A nylon membrane. Transcript levels of TLR4 and actin were detected using biotin-labeled cDNA probes. (C) BAL macrophages were collected from WT, PU/ER(T)+/−, and PU/ER(T)+/+ bone marrow chimera mice on the 45th and 55th day (before and after clearance of circulating tamoxifen), and protein lysates were prepared. Expression of TLR4 protein in alveolar macrophages was determined by Western blotting analysis. Blots presented in panels B and C are representatives of samples analyzed from each group (n = 4).
Effect of PU.1 deficiency on TLR4 expression in WT, PU/ER(T)+/−, and PU/ER(T)+/+ bone marrow chimera mice. (A) BAL macrophages were collected from WT, PU/ER(T)+/−, and PU/ER(T)+/+ bone marrow chimera mice on the 45th and 55th day (before and after clearance of circulating tamoxifen), and then total RNA was isolated. Expression levels of TLR4 were measured by qRT-PCR. Data are representative of the average of measurements from 4 different mice. (B) Total RNA isolated as described in panel A was electrophoresed and transferred on to Biodyne A nylon membrane. Transcript levels of TLR4 and actin were detected using biotin-labeled cDNA probes. (C) BAL macrophages were collected from WT, PU/ER(T)+/−, and PU/ER(T)+/+ bone marrow chimera mice on the 45th and 55th day (before and after clearance of circulating tamoxifen), and protein lysates were prepared. Expression of TLR4 protein in alveolar macrophages was determined by Western blotting analysis. Blots presented in panels B and C are representatives of samples analyzed from each group (n = 4).
Discussion
PU.1 was first identified because of its involvement in the multiple hematopoietic abnormalities34 and pathogenesis of erythroleukemia35 and has been implicated in both lymphoid and myeloid lineage development.36-38 Bone marrow precursor cells from PU.1 null mice can be induced by treatment with GM-CSF to develop some of the phenotypic characteristics of monocytic precursor cells, but further development beyond a stage of monocytic precursors or embryonic macrophages and expression of surface CD 11b is blocked.39,40 Pulmonary macrophages express CD 11b that is highly dependent on expression of the transcription factor PU.1 that also is involved in transcription of genes governing the initiation and intensity of endotoxin responsiveness, including TREM1,41 TLR2,14 and TLR4.15 These genes are associated with terminal macrophage differentiation and therefore may play an essential role in governing the initiation and intensity of the endotoxin response.13,14 In humans subjected to experimental endotoxemia, there is a 2- to 3-fold increase in CD 11b, TLR4, and TLR242 expression in circulating blood monocytes, suggesting the involvement of PU.1 in inflammatory signaling.
In spite of a growing body of in vitro data and reports of human sample studies, there is no direct in vivo evidence on how PU.1 influences the immune-effector function of mature macrophages, because the PU.1 null mice are embryonically lethal. Therefore, we aimed to elucidate the role of PU.1 in regulating lung and systemic inflammation by using a novel bone marrow chimera mouse that displays a conditional PU.1 knockout phenotype in mature macrophages. To our knowledge, this is the first report that demonstrates the role of the lineage-specific transcription factor PU.1 in mature macrophages, in the context of an acute model of lung and systemic inflammation using PU/ER(T) bone marrow chimera mice. Previous studies showed that the depletion of PU.1 resulted in increased susceptibility to sepsis presumably because of defective maturation of myeloid cells.20 However, there was lack of information about the functional significance of PU.1, once macrophages are fully matured. In the present study, we found that attenuated systemic and lung inflammation was associated with PU.1 deficiency in bone marrow chimera mice during endotoxin treatment (Figure 5A-E). The attenuation in inflammatory reaction was not because of the difference in number of alveolar macrophages or their death in PU.1-deficient mice (Figures 4B and 5C). It is interesting to note that the expression of immature macrophage surface makers such as ER-MP58 and Ly-6C is not significantly different in WT and heterozygotes (Figure 3A). Apoptosis assay of BAL fluid cells showed that in the absence of functional PU.1, the alveolar macrophages were alive, not apoptotic (Figure 4B). This observation suggests that PU.1 is important in differentiation of macrophages from hematopoietic cells, but it is dispensable for survival, once macrophages are fully differentiated. Importantly, the depletion of PU.1 in mature macrophages resulted in blunted immune response to endotoxin challenge that in turn improved survival in endotoxin induced septic shock (Figure 3D). In the present study, the depletion of PU.1 in mature macrophages lead to less activation of NF-κB, as measured by bioluminescence and organ-specific NF-κB reporter activity (Figure 5A-B). Furthermore, there was marked impairment of neutrophil recruitment into lungs in response to endotoxin in PU.1-deficient bone marrow chimeras (Figure 5C). Consistent with these findings, the serum levels of NF-κB–dependent chemokines and cytokines were decreased in PU/ER(T) homo- and heterozygotic bone marrow chimera mice (Figure 5E). Less recruitment of neutrophils in the lungs could be because of decreased secretion of neutrophilic chemokine KC. However, some studies also suggested that PU.1-depleted neutrophils are arrested in premature stage of development and are not fully functional.43
Our previous studies showed that PU.1 plays an important role in enhancing the endotoxin response through increasing inflammatory gene expression (eg, COX-2 and lipocalin-type prostaglandin D synthase), through combinatorial interaction with other transcription factors that include C/EBPβ,44 activator protein (AP)–1 (c-Jun),45 and NF-κB–inducing kinase.46 We also have reported that small interfering RNA knockdown of PU.1 down-regulates TLR4, MIP-1, and COX-2 in the RAW 264.7 cell line.47 TLR4 is important for endotoxin response signaling in macrophages, and its stringent regulation is required for the appropriate innate immune system activation to prevent septic shock. Studies by other researchers reported the transcriptional regulation of TLR4 by PU.1.16 Our observations using the PU.1-deficient chimera mice also indicated the dependence of TLR4 expression on PU.1, thereby affecting the LPS responses in chimera mice (Figure 6A-C). Observations from the current study and our previous work clearly indicate that PU.1 is associated with markers of M1 macrophage phenotype (COX-2, iNOS, and also inflammatory cytokines such as IL-6 and TNF-α) but do not seem to affect the M2 macrophage markers (CD 206, arginase 1, or IL-10).
In a recent study, inducible p300 binding to chromatin was used to identify enhancers controlling endotoxin-stimulated gene expression in macrophages. Among the identified enhancers, binding sites for the lineage-restricted and constitutive Ets protein PU.1 coexisted with those for ubiquitous stress-inducible transcription factors such as NF-κB, IFN regulatory factor, and AP-1.17-19 Also, studies involving genome-wide chromatin analysis indicated that PU.1 sets the stage for the activity of ubiquitous transcription factors activated by inflammatory stimuli, such as NF-κB, AP-1, and IFN regulatory factors.17 Specifically, the intersection of lineage-determining and stimulus-activated transcription factors at gene enhancer regions explains cell-type specificity in inflammatory responses.17 These findings support the emerging new regulatory mechanisms of PU.1-associated inflammatory response that depend together on the stimuli, cell type, and other interacting transcription factors.
In summary, we have identified the mechanistic role of PU.1 in mediating mature macrophage inflammatory phenotype in a murine model of endotoxin-induced systemic inflammation. We established a bone marrow chimera mice model with functional PU.1 knockout phenotype in mature macrophages. In these mice, NF-κB activation was markedly attenuated in different organs, compared with WT mice. Myeloperoxidase in lung tissue was decreased significantly associated with a decrease in neutrophilic infiltration. PU/ER(T)+/− heterozygotes showed less lung inflammation and better survival on challenge with endotoxin, indicating a direct link between the lineage-specific transcription factor PU.1 and inflammation. These findings indicate that NF-κB–mediated neutrophilic lung and systemic inflammation is reduced in mice with macrophages that lack functional PU.1. To our knowledge this is the first report that provides direct evidence on the in vivo role of PU.1 in generating endotoxin induced systemic and lung inflammation in response to endotoxemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the National Institutes of Health (grants R01 HL075557, HL 103643, and T32 HL082547) and a Department of Veterans Affairs merit review grant (1I01BX000108, J.W.C.).
National Institutes of Health
Authorship
Contribution: M.K. performed Southern blotting, generated bone marrow chimeras, and analyzed lung injury parameters; characterized the immunophenotype of mice; and wrote the manuscript; X.W. performed flow cytometry and generated bone marrow chimeras; J.D. analyzed in vivo bioluminescence of chimera mice; H.P. analyzed cells by flow cytometry; J.W.C. designed the research study, secured funding, and interpreted the results; G.Y.P. edited the manuscript; E.W.S. provided the PU/ER(T) transgenic mice; and U.A.M., R.T.S., R.S.F., and L.X. participated in planning the study.
Correspondence: John W. Christman, Section of Pulmonary, Critical Care, Sleep, and Allergy, Department of Medicine, University of Illinois at Chicago, 840 South Wood St, Chicago, IL 60612; e-mail: jwc@uic.edu.
References
Author notes
M.K. and X.W. share primary authorship.

![Figure 1. Genotypic and phenotypic characterization of PU/ER(T) mice. (A) Schematic illustration of the PU/ER(T) targeting vector map that includes the vector, the PU.1 locus, and the targeted PU.1 mutant allele generated by homologous recombination. The coding sequence for the PU/ER(T) transgene is knocked into the WT PU.1 loci (exon 1 harboring the nuclear localization signal sequence) as illustrated. Double-headed arrow corresponds to the sequence used as a probe for Southern hybridization that encompasses a part of exon 1 its 5′ sequence. The restriction sites are indicated as E1, EcoRI; H3, HindIII. (B) Southern blot analysis of WT, homozygote [PU/ER(T)+/+], and heterozygote [PU/ER(T)+/−] fetal liver genomic DNA that designates the location of the 8.3-kb WT, 8.3- and 5.60-kb PU/ER(T)+/−, and 5.6-kb PU/ER(T)+/+ bands. (C) Flow cytometry analysis of day 14 ± 1 FLC suspension from PU/ER(T)+/− mice. Numbers in the bottom right quadrates indicate percentage of cells carrying the CD 11b surface antigen. Data are representative of 3 individual measurements.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/19/10.1182_blood-2011-03-341123/4/m_zh89991181610001.jpeg?Expires=1767898530&Signature=Rtgog6321o7kBnAfYKWOaY4CYFBNPv0CPldzvVYUwCgZ826nB6cP2aiPeYjTkiSanH7lt7gHSKfvGYaWbVPD2mcmMfvntYEI-faTd43vki6CDhWDP-LLCWlmpNCL7x~V2QwGFmx2ByCZ4qzxVOUFRcJRvwldTZPRKr~po1I8ZQCuQD6xvNfepe5R7CB34e1T~tzlsTqJxBH-o8necfi-CHuWpp7aQdJG~h0iA3CWIyyxRxte44l~4YKApfwNgDarU8kBq8oeyTMa~SB~QJl8cI4ECi1PNRqrZWDSMEc40di3WOFPJ2Y2uKh7Tqc9NIttCjXI-y0ci6uaZt75x7~~LA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)