Abstract
Mastocytosis is a heterogeneous disease characterized by the accumulation of mast cells in one or more organs. Our objective was to identify a peripheral mast cell precursor and assess its variation rate in mastocytosis. A peripheral blood phenotypic analysis was performed among 50 patients with mastocytosis who were enrolled in a prospective multicentric French study, and the phenotypic analysis results of the patients were compared with those of healthy donors. The rate of peripheral blood CD34−c-Kit+ cells correlated with the severity of mastocytosis. This cellular population was isolated from healthy donors as well as from patients with systemic mastocytosis. After 30 days of culture, the CD34−c-Kit+ cells gave birth to mature mast cells, indicating that this cellular population constitutes a mast cell circulating precursor. Monitoring peripheral CD34−c-Kit+ cells by flow cytometry could be a useful and low-invasive tool to determine the disease severity and the relapses and to assess treatment efficiency.
Introduction
Mastocytosis is a rare heterogeneous disease characterized by the accumulation of mast cells (MCs) in one or several organs.1-6 A World Health Organization (WHO) classification described several disease subcategories, broadly divided into localized versus systemic disease. Systemic mastocytosis (SM) is subsequently divided into indolent and aggressive disease based on damage to organs.6-8 The c-Kit tyrosine kinase receptor is expressed on MCs. Adults with SM usually present c-Kit mutations, most frequently D816V, which allows MC survival and accumulation and activation. Recently, flow cytometric studies showed that pathologic MCs from patients with mastocytosis, in addition to c-Kit expression, display unique aberrant immunophenotypic characteristics compared with normal MCs.9-14 Normal MCs are derived from CD34−c-Kit+ blood precursors, which terminally differentiate in peripheral tissues on SCF stimulation.15
The aim of our study was to detect CD34c-Kit+ cells in peripheral blood (PB) of patients with mastocytosis and investigate whether these cells could give birth to mature MCs in the presence or absence of SCF as well as to investigate the relevance of CD34−c-Kit+ cell detection for positive diagnosis, classification, and follow-up.
Methods
Patients and data collection
Fifty consecutive patients (26 men and 24 women) with a mastocytosis diagnosis as defined by the WHO criteria6 and 15 healthy donors were enrolled in a prospective national multicenter study from 1999 to 2008. Mastocytosis subcategories were composed of cutaneous (n = 4), indolent SM (ISM, n = 25), aggressive SM (ASM, n = 16), and SM with associated hematologic non-MC-lineage disease (SM-AHNMD; n = 5). The median age of phenotype was 52 years (range, 7-75 years). Three patients (patients 32, 38, and 42) were followed at each hospitalization. A PB phenotypic analysis was performed, and the results were correlated with clinical manifestations and treatment responses. All patients were included in the Mastocytosis Pathophysiological Study, sponsored by the Association for the Initiative and Research of Mastocyte and Mastocytosis, which began in 2003. The study was approved by the Necker Hospital Ethical Committee and was carried out in accordance with the Helsinki convention. Each patient provided an informed consent. The c-Kit gene sequencing was performed for each patient in skin, blood, and/or bone marrow. The D816V mutation of c-Kit was detected among 35 patients, including patients 32, 38, and 42. Among them, 13 were c-Kit wild-type (WT; Table 1).
Main features of the study population
| Patient no. . | Age at phenotype, y . | Tryptase, μg/L . | Valent stage . | c-Kit mutation . |
|---|---|---|---|---|
| 1 | 44 | 20 | CM | D-816-V |
| 2 | 39 | 5 | CM | WT |
| 3 | 49 | 1 | CM | WT |
| 4 | 12 | 5 | CM | WT |
| 5 | 57 | 45 | ISM | D-816-V |
| 6 | 63 | 50 | ISM | D-816-V |
| 7 | 67 | 9 | ISM | D-816-V |
| 8 | 26 | 22 | ISM | D-816-V |
| 9 | 75 | 124 | ISM | D-816-V |
| 10 | 40 | 153 | ISM | WT |
| 11 | 52 | 93 | ISM | D-816-V |
| 12 | 51 | 21 | ISM | WT |
| 13 | 65 | 318 | ISM | D-816-V |
| 14 | 49 | 40 | ISM | D-816-V |
| 15 | 43 | 16 | ISM | D-816-V |
| 16 | 60 | 140 | ISM | D-816-V |
| 17 | 75 | 2 | ISM | D-816-V |
| 18 | 57 | 48 | ISM | WT |
| 19 | 37 | 11 | ISM | D-816-V |
| 20 | 37 | 50 | ISM | D-816-V |
| 21 | 30 | 49 | ISM | WT |
| 22 | 55 | 229 | ISM | WT |
| 23 | 23 | 100 | ISM | D-816-V |
| 24 | 39 | 6 | ISM | D-816-V |
| 25 | 72 | 822 | ISM | D-816-V |
| 26 | 18 | 28 | ISM | D-816-V |
| 27 | 62 | 33 | ISM | D-816-V |
| 28 | 30 | 68 | ISM | D-816-V |
| 29 | 26 | 152 | ISM | D-816-V |
| 30 | 45 | 17 | ASM | D-816-V |
| 31 | 38 | 233 | ASM | WT |
| 32 | 70 | 421 | ASM | D-816-V |
| 33 | 72 | 304 | ASM | D-816-V |
| 34 | 25 | 228 | ASM | D-816-V |
| 35 | 36 | 49 | ASM | D-816-V |
| 36 | 7 | 734 | ASM | D-816-V |
| 37 | 42 | 15 | ASM | D-816-V |
| 38 | 68 | 304 | ASM | D-816-V |
| 39 | 70 | 330 | ASM | WT |
| 40 | 43 | 3 | ASM | WT |
| 41 | 74 | 156 | ASM | D-816-V |
| 42 | 49 | 39 | ASM | D-816-V |
| 43 | 55 | 95 | ASM | WT |
| 44 | 64 | 5 | ASM | ND |
| 45 | 32 | 356 | ASM | D-816-V |
| 46 | 72 | 200 | SM-AHNMD | D-816-V |
| 47 | 52 | 62 | SM-AHNMD | D-816-V |
| 48 | 54 | 12 | SM-AHNMD | WT |
| 49 | 40 | 10 | SM-AHNMD | D-816-V |
| 50 | 73 | 98 | SM-AHNMD | D-816-V |
| Patient no. . | Age at phenotype, y . | Tryptase, μg/L . | Valent stage . | c-Kit mutation . |
|---|---|---|---|---|
| 1 | 44 | 20 | CM | D-816-V |
| 2 | 39 | 5 | CM | WT |
| 3 | 49 | 1 | CM | WT |
| 4 | 12 | 5 | CM | WT |
| 5 | 57 | 45 | ISM | D-816-V |
| 6 | 63 | 50 | ISM | D-816-V |
| 7 | 67 | 9 | ISM | D-816-V |
| 8 | 26 | 22 | ISM | D-816-V |
| 9 | 75 | 124 | ISM | D-816-V |
| 10 | 40 | 153 | ISM | WT |
| 11 | 52 | 93 | ISM | D-816-V |
| 12 | 51 | 21 | ISM | WT |
| 13 | 65 | 318 | ISM | D-816-V |
| 14 | 49 | 40 | ISM | D-816-V |
| 15 | 43 | 16 | ISM | D-816-V |
| 16 | 60 | 140 | ISM | D-816-V |
| 17 | 75 | 2 | ISM | D-816-V |
| 18 | 57 | 48 | ISM | WT |
| 19 | 37 | 11 | ISM | D-816-V |
| 20 | 37 | 50 | ISM | D-816-V |
| 21 | 30 | 49 | ISM | WT |
| 22 | 55 | 229 | ISM | WT |
| 23 | 23 | 100 | ISM | D-816-V |
| 24 | 39 | 6 | ISM | D-816-V |
| 25 | 72 | 822 | ISM | D-816-V |
| 26 | 18 | 28 | ISM | D-816-V |
| 27 | 62 | 33 | ISM | D-816-V |
| 28 | 30 | 68 | ISM | D-816-V |
| 29 | 26 | 152 | ISM | D-816-V |
| 30 | 45 | 17 | ASM | D-816-V |
| 31 | 38 | 233 | ASM | WT |
| 32 | 70 | 421 | ASM | D-816-V |
| 33 | 72 | 304 | ASM | D-816-V |
| 34 | 25 | 228 | ASM | D-816-V |
| 35 | 36 | 49 | ASM | D-816-V |
| 36 | 7 | 734 | ASM | D-816-V |
| 37 | 42 | 15 | ASM | D-816-V |
| 38 | 68 | 304 | ASM | D-816-V |
| 39 | 70 | 330 | ASM | WT |
| 40 | 43 | 3 | ASM | WT |
| 41 | 74 | 156 | ASM | D-816-V |
| 42 | 49 | 39 | ASM | D-816-V |
| 43 | 55 | 95 | ASM | WT |
| 44 | 64 | 5 | ASM | ND |
| 45 | 32 | 356 | ASM | D-816-V |
| 46 | 72 | 200 | SM-AHNMD | D-816-V |
| 47 | 52 | 62 | SM-AHNMD | D-816-V |
| 48 | 54 | 12 | SM-AHNMD | WT |
| 49 | 40 | 10 | SM-AHNMD | D-816-V |
| 50 | 73 | 98 | SM-AHNMD | D-816-V |
ND indicates not done.
PBMC isolation, flow cytometric analysis, and cell culture
Blood was collected on heparinized tubes, and PBMCs were isolated by Ficoll-IPaque gradient (GE Healthcare). PBMCs were stained using: CD34 conjugated to FITC and CD117/c-Kit to PE. Control isotype-matched antibodies were used at appropriate concentration (Beckman Coulter). After washing, 2 × 104 events were analyzed by FACSCalibur (BD Biosciences). The c-Kit+ cells were sorted after c-Kit-allophycocyanin staining associated with allophycocyanin-coated magnetic beads (Miltenyi Biotec). The c-Kit+ sorted cells were stained with CD34-FITC, c-Kit-allophycocyanin, and CD45-peridinin chlorophyll protein (BD Biosciences). The c-Kit+CD34−CD45low cells were sorted on a FACSAria sorter (BD Biosciences) and cultured on 96-well plates in 200 μL of IMDM supplemented16 for 30 days with or without SCF adjunction (50 μg/mL). The medium was renewed twice a week. For each cytospin, 100 μL of PBS/2% FBS containing 10 000 sorted cells was used and stained with May-Grünwald-Giemsa at culture day 0 and 30. The histamine was measured in the supernatant as previously described.17
D816V c-Kit mutation detection and serum tryptase measurement
The D816V c-Kit mutation was determined as previously described.18,19 Total serum tryptase (protryptase + β-tryptase) was measured using fluorescent enzyme-linked immunoassay (Unicap; Pharmacia).20 The detection limit of this assay is 1 ng/mL, and in healthy controls, serum tryptase levels range between less than 1 and 15 ng/mL, with a median of approximately 5 ng/mL.21
Statistical analysis
Statistical comparisons between characteristics of healthy donors and patients (cutaneous mastocytosis [CM], ISM, ASM, and SM-AHNMD) were based on unpaired t test. All reported P values were 2-tailed with 95% CI; P < .05 was considered significant. Data were analyzed using GraphPad Prism Version 5.01 software (GraphPad Software).
Results and discussion
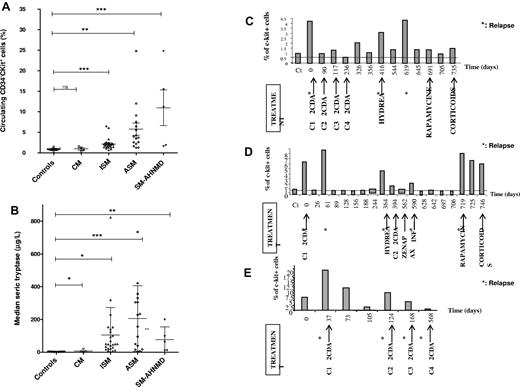
The rate of the circulating CD34−c-Kit+ cell population was significantly higher among the SM subcategory patients (all P < .005) compared with the healthy donors and CM patients (Figure 1A-B). The rate was more elevated among patients with ASM and SM-AHNDM than those with indolent forms of SM. Serum tryptase rate analysis revealed that tryptase was significantly higher in all mastocytosis subcategories (CM, P = .0385; ISM, P = .0242; ASM, P = .0005; and SM-AHNMD, P = .0012). The number of CD34−c-Kit+ cells was assessed during disease evolution and under various treatments of 3 ASM patients (Figure 1C-E). These results showed that the percentage of CD34−c-Kit+ cells decreased 24 to 48 hours after each efficient treatment and increased after each relapse, preceding serum tryptase level modification. Taken together, these results demonstrate that a circulating CD34−c-Kit+ cell population exists among patients with mastocytosis, and the population rate correlated with mast cell burden, with higher elevation rates among SM subcategories compared with CM subcategories and healthy donors (P = .0146 vs P = .178). This CD34−c-Kit+ cell population was markedly higher among patients with the most aggressive SM subcategories, such as ASM and SM-AHNMD. These more aggressive SM forms are associated with poor prognosis and shortened life expectancy.22,23 The CD34−c-Kit+ cell population number correlated to cytoreductive treatment efficiency and could predict clinical disease relapses (Figure 1C-E).
Clinical applications of circulating CD34− c-Kit+ population rate. (A-B) Correlation of Valent stage disease with the rate of circulating CD34−c-Kit+ population and the rate of serum tryptase. (A) The rate of the circulating CD34−c-Kit+ is shown for each patient along with disease stage, indicating the aggressiveness of their disease, and compared with healthy controls. All mastocytosis patients with systemic forms had a significantly higher rate of CD34−c-Kit+ cells than the controls: ISM (P = .0007), ASM (P = .0031), and SM-AHNMD (P = .0005). This association was not found for cutaneous forms, which were comparable with the healthy controls (P = .5). (B) The serum rate of tryptase was always elevated among patients with mastocytosis, independently from the stage of the disease: cutaneous as well as systemic forms had an elevated serum tryptase. Indeed, serum tryptase levels among controls were always lower than patients with CM (P = .0385), ISM (P = .0242), ASM (P = .0005), or SM-AHNMD (P = .0012). (C-E) Clinical and biologic follow-up of 3 patients with aggressive systemic mastocytosis until death. (C) Patient 42. (D) Patient 32. (E) Patient 38. The clinical evolution and treatment are mentioned as well as the rate of the circulating CD34−c-Kit+ population. It shows that, when patients present aggressive disease with massive mast cell infiltration, the rate of the circulating CD34−c-Kit+ population is a good tool to quickly follow (within 24-48 hours) the clinical evolution of the disease and to determine the efficiency of the treatments. CT indicates healthy control; 2CDA, Leustatin, Zenapax, daclizumab; and C1 to C4, cure number.
Clinical applications of circulating CD34− c-Kit+ population rate. (A-B) Correlation of Valent stage disease with the rate of circulating CD34−c-Kit+ population and the rate of serum tryptase. (A) The rate of the circulating CD34−c-Kit+ is shown for each patient along with disease stage, indicating the aggressiveness of their disease, and compared with healthy controls. All mastocytosis patients with systemic forms had a significantly higher rate of CD34−c-Kit+ cells than the controls: ISM (P = .0007), ASM (P = .0031), and SM-AHNMD (P = .0005). This association was not found for cutaneous forms, which were comparable with the healthy controls (P = .5). (B) The serum rate of tryptase was always elevated among patients with mastocytosis, independently from the stage of the disease: cutaneous as well as systemic forms had an elevated serum tryptase. Indeed, serum tryptase levels among controls were always lower than patients with CM (P = .0385), ISM (P = .0242), ASM (P = .0005), or SM-AHNMD (P = .0012). (C-E) Clinical and biologic follow-up of 3 patients with aggressive systemic mastocytosis until death. (C) Patient 42. (D) Patient 32. (E) Patient 38. The clinical evolution and treatment are mentioned as well as the rate of the circulating CD34−c-Kit+ population. It shows that, when patients present aggressive disease with massive mast cell infiltration, the rate of the circulating CD34−c-Kit+ population is a good tool to quickly follow (within 24-48 hours) the clinical evolution of the disease and to determine the efficiency of the treatments. CT indicates healthy control; 2CDA, Leustatin, Zenapax, daclizumab; and C1 to C4, cure number.
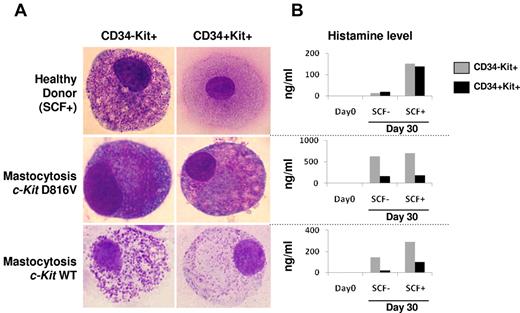
CD34−c-Kit+ cells were isolated from healthy donors (n = 3) and patients (n = 4) and cultured in a medium supplemented or not with SCF. Among the patients, 3 were bearing the D816V c-Kit mutation and 1 was c-Kit WT. After 30 days of culture in the presence of SCF, mature MCs were identified by morphology and histamine secretion (Figure 2). In the absence of SCF, only MCs derived from patients with mastocytosis (bearing WT c-Kit or D816V mutation) were able to differentiate into mature MCs, in contrast to healthy donors. This result suggested that the MC precursor from all patients with mastocytosis could mature, independently from SCF.
Cytologic aspects (May-Grünwald-Giemsa smears) and histamine production of isolated CD34+/−c-Kit+/− compartments from PB of patients with mastocytosis and healthy controls. (A) Among healthy donors, cultures of both CD34+c-Kit+ and CD34−c-Kit+ cell subsets showed differentiation into mature mast cells in the presence of SCF (top panel), whereas no differentiation was observed in the absence of SCF (data not shown). In patients with proven mastocytosis, bearing either the classic D816V c-Kit mutation or WT c-Kit, similar differentiation into mature mast cells was observed in the presence or absence of SCF (bottom panel). (B) Histamine dosage also supported mast cell differentiation, demonstrating increased histamine levels with or without SCF in both cultured subsets from the patients with mastocytosis, whereas among healthy controls, histamine levels increased only in the presence of SCF.
Cytologic aspects (May-Grünwald-Giemsa smears) and histamine production of isolated CD34+/−c-Kit+/− compartments from PB of patients with mastocytosis and healthy controls. (A) Among healthy donors, cultures of both CD34+c-Kit+ and CD34−c-Kit+ cell subsets showed differentiation into mature mast cells in the presence of SCF (top panel), whereas no differentiation was observed in the absence of SCF (data not shown). In patients with proven mastocytosis, bearing either the classic D816V c-Kit mutation or WT c-Kit, similar differentiation into mature mast cells was observed in the presence or absence of SCF (bottom panel). (B) Histamine dosage also supported mast cell differentiation, demonstrating increased histamine levels with or without SCF in both cultured subsets from the patients with mastocytosis, whereas among healthy controls, histamine levels increased only in the presence of SCF.
Previous studies reporting MC immunophenotype on large patient cohorts were usually performed on BM MCs, as recommended.10,12,14 Our results on a large patient cohort show that PB phenotype could be useful, in addition to BM aspiration, to study MC phenotype among patients with mastocytosis. Further, the PB phenotype could represent an alternative from BM aspiration to follow disease activity or to monitor treatment efficiency because the result of PB phenotype is fast, reproducible, and more sensitive than serum tryptase measurement.
In addition, our findings give insight to the pathophysiology of mastocytosis and normal MC lineage development by providing strong evidence that MC accumulation in various tissues originates from the amplification of clonal blood-circulating precursors that are able to differentiate and survive independently from SCF because of constitutive activation of c-Kit as a consequence of c-Kit mutations or other unknown oncogenic events. These data may open new avenues of research in the field of MC precursors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the following physicians for collecting patient data: Drs M. Blanc (Chambèry), D. Bordessoule (Limoges), O. Fain (Bondy), C. Hoarau (Tours), H. Coignard (Paris), F. Suarez (Paris), and C. Roux-Serratrice (Marseille). The authors also thank François Machavoine, Fabienne Palmérini, Elke Schneider, and Françoise Valensi for technical assistance and helpful discussions.
This work was supported in part by Inserm, la Ligue Nationale Contre le Cancer (Equipe labellisée, P.D. and O.H.), Agence Nationale pour la Recherche (grant Maladies Rares, P.D. and O.H.), Fondation pour la Recherche Médicale (P.D. and O.H.), and Institut National du Cancer (P.D. and O.H.). S.G.-L. was supported by Fondation pour la Recherche Médicale. S.B. was supported by Société Française de Dermatologie.
Authorship
Contribution: S.G.-L. wrote the manuscript, carried out experiments, and collected, analyzed, and interpreted data; Y.L. designed the study, carried out experiments, collected, analyzed, and interpreted data, and assisted in writing the manuscript; O.H. designed the study, interpreted data, recruited patients, assisted in writing, and finalized the manuscript; P.D., F.F., and S.B. carried out experiments, collected, analyzed, and interpreted data, and assisted in writing the manuscript; C.B., J.B. K.H., and L.L. carried out experiments and collected and analyzed data; and M.-O.C., J.-M.L., C.d.G., G.D., F.L., M.H., and O.L. helped design the study, edited the manuscript, and recruited patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olivier Hermine, Service d'Hématologie Adultes, CNRS UMR 8147 et Centre de Référence sur les Mastocytoses, Hôpital Necker-Enfants Malades, Université Paris Descartes, AP-HP, 149 Rue des Sèvres, 75743 Paris Cedex 15, France; e-mail: ohermine@gmail.com.
References
Author notes
S.G.-L., L.L., and C.B. contributed equally to this study.
Y.L. and O.H. codirected this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal