Abstract
Recipients of umbilical cord blood (UCB) transplantation (UCBT) face a high risk of morbidity and mortality related to opportunistic infections (OI) and leukemic relapse. To understand the molecular basis of these UCBT-related complications, the characteristics of UCB-derived antigen-specific CD8+ T cells were examined in a group of pediatric UCBT recipients. Compared with the UCB graft inoculum and the late post-UCBT period (12-36 months), declining clonal diversity of UCB-derived CD8+ T cells specific for the Melan-A26-35 A27L peptide and high frequencies of PD-1-expressing CD8+ T cells were observed in the first 3 months after UCBT, a period during which OIs are most frequent. The CD8+ T-cell compartment predominantly comprised CD45RA+ CCR7− terminally differentiated effector-memory T cells until 6 months after UCBT, at which time the polyfunctionality of antigen-specific CD8+ T cells was reestablished. Finally, the frequency of PD-1+ CD8+ T cells was significantly higher in subjects who subsequently experienced leukemic relapse. This study informs the biologic properties of UCB-derived CD8+ T cells and provides a rationale for the characteristics of UCBT in terms of immune reconstitution and OI. These results also suggest that the elevated frequency of PD-1+ CD8+ T cells could be associated with leukemic relapse in pediatric UCBT recipients.
Introduction
Umbilical cord blood (UCB) transplantation (UCBT) is commonly used to treat a variety of hematologic diseases in children.1,2 A reduced incidence of graft versus host disease (GVHD) compared with BM transplantation (BMT) was documented after UCBT.3 UCBT was associated with slow engraftment, delayed CD8+ T-cell recovery, a high incidence of graft failure, and a high incidence of opportunistic infections (OIs) in the first 3 to 6 months after transplantation.4-6 Approximately 20% of pediatric UCBT recipients will also experience leukemic relapse, a frequency similar to that observed in BMT.7
In the context of BMT, graft-derived CD8+ T cells are known to shield the graft recipient against OI and leukemic relapse.8,9 UCBT involves the transfer of multiple cell subtypes into the recipient, including large amounts of CD8+ T lymphocytes. Although the presence of UCB-derived CD8+ T cells in the graft inoculums can provide some degree of protection against OI and leukemic relapse to UCBT recipients,10,11 recent studies suggest that these cells might only mediate minimal levels of antiviral immunity.12,13 The relative inefficacy of UCB-derived CD8+ T cells to prevent OI and their failure to prevent leukemic relapse in some patients is not well understood.
On the basis of cell-surface expression of CD45RA and CCR7, CD8+ T cells can be classified as naive, central memory, effector memory, and terminally differentiated.14 Using peptide-MHC tetramers, antigen-specific CD8+ T cells isolated from UCB were shown to be mostly naive and clonally diversified. In addition, they produced significantly less IFN-γ but were more likely to achieve terminal differentiation and less likely to exhibit bifunctional properties (IFN-γ production and cytolytic activity) than T lymphocytes derived from adult blood.15 In theory, these properties are compatible with a low incidence of GVHD and a high incidence of OI; however, little is known regarding the fate of CD8+ T cells once they are transplanted into UCBT recipients.
The objective of the present study was to characterize the reconstitution of CD8+ T cells in pediatric UCBT recipients to address a series of questions: (1) Do UCB-derived CD8+ T cell clones persist during immune reconstitution? (2) To what extent do they participate in the composition of the reconstituted T-cell repertoire? (3) How does clonal diversity of antigen-specific CD8+ T cells develop? (4) How do they differentiate in terms of naive-effector-memory phenotype and expression of T-cell exhaustion markers? (5) How does their polyfunctionality, which reflects their efficacy to control chronic viral infections,16 evolve in the post-UCBT period? We examined CD8+ T cells specific for the HLA-A2–restricted Melan-A26-35 A27L peptide (A2/Melan-A)17 as a model for the reconstitution of an antigen-specific repertoire. A2/Melan-A–specific T cells represent one of the only preimmune T-cell repertoires that can be studied in humans.15,17,18 Here, we provide evidence that some UCB-derived CD8+ T lymphocytes persisted in pediatric recipients in the first 6 months after UCBT. Their clonal diversity reached a nadir at 3 months after UCBT. At that point, the CD8+ compartment was predominantly composed of terminally differentiated T cells, and both CD4+ and CD8+ subsets were enriched in cells that expressed programmed death 1 (PD-1). From 6 months after UCBT, the CD8+ T-cell repertoire was progressively restored with a naive, diversified, and polyfunctional population. Finally, higher frequencies of PD-1+ CD8+ T cells were detected at 2 and 6 months after UCBT in subjects who subsequently experienced leukemic relapse, which suggests that PD-1 expression on CD8+ T cells could be associated with this complication.
Methods
Study subjects
This study was approved by the Institutional Review Board of Centre hospitalier universitaire (CHU) Sainte-Justine, Montreal, QC, and was conducted according to the standards described in the Helsinki Declaration of 1975, as revised in 2000. Full informed consent was obtained from all study participants and their parents or legal guardians. Pediatric HLA-A2+ subjects who underwent UCBT for the treatment of hematologic or neoplastic diseases were enrolled at CHU Sainte-Justine between October 2004 and December 2009 (n = 26). Median age at study entry was 5.42 years (range = 0.33-17.0 years). UCB and venous blood samples (2-10 mL) were obtained from the graft inoculums and from transplanted subjects at 1, 2, 3, 6, 12, 18, 24, and 36 months after UCBT. UCB units used in transplant procedures were obtained from national and international UCB repositories. All UCB units used in these patients were HLA-A2+ and had a 4/6 or greater HLA-A, HLA-B, and HLA-DRB1 allele-level match with the recipient. UCB mononuclear cells (UCBMCs) and PBMCs were isolated on Ficoll-Hypaque gradients (Amersham Biosciences) and cryopreserved in 90% vol/vol FBS (Invitrogen) supplemented with 10% vol/vol DMSO. All subjects received myeloablative conditioning regimens, including total-body irradiation (TBI; 1200 cGy fractionated in 8 doses) or busulfan (0.8 mg/kg, fractionated in 16 doses). GVHD prophylaxis consisted of cyclosporin A and corticosteroids. Subjects were infused with antithymocyte globulin (ATG) 2 mg/kg (Thymoglobulin; Sangstat) on days −2, −1, +1, and +2 relative to the UCBT procedure. Granulocyte-colony stimulating factor (G-CSF) was also administered starting on day +1. Two subjects were lost to follow-up. The transplant procedure was detailed in Dalle et al.19 Clinical characteristics of study subjects are summarized in Table 1.
Clinical characteristics of transplanted patients
| . | n . | % . | Patient reference ID . |
|---|---|---|---|
| Sex | |||
| Female | 11 | 42.3 | 1, 5, 7, 8, 11, 12, 20, 21, 22, 23, 25 |
| Male | 15 | 57.7 | 2, 3, 4, 6, 9, 10, 13, 14, 15, 16, 17, 18, 19, 24, 26 |
| UCB graft | |||
| Single unit | 24 | 92.3 | All except 19, 24 |
| Double units | 2 | 7.7 | 19, 24 |
| Hematologic malignancies | |||
| ALL | 11 | 42.3 | 1, 3, 8, 10, 13, 14, 15, 17, 18, 23, 26 |
| MDS | 8 | 30.8 | 5, 6, 7, 9, 12, 21, 22, 24 |
| AML | 4 | 15.4 | 2, 4, 20, 25 |
| Others | |||
| FHLH | 1 | 3.8 | 11 |
| Farber disease | 1 | 3.8 | 16 |
| Hurler syndrome | 1 | 3.8 | 19 |
| HLA disparity | |||
| 0 | 9 | 34.6 | 1, 4, 5, 6, 12, 14, 19, 23, 25 |
| 1 | 9 | 34.6 | 2, 3, 8, 10, 11, 16, 20, 21, 26 |
| 2 | 8 | 30.8 | 7, 9, 13, 15, 17, 18, 22, 24 |
| Outcome | |||
| Death | 8 | 30.8 | 1, 2, 7, 8, 12, 14, 19, 23 |
| Cytomegalovirus infection | 3 | 11.5 | 7, 9, 12 |
| Herpes virus infection (other) | 9 | 34.6 | 2, 9, 10, 11, 13, 15, 17, 21, 25 |
| Leukemic relapse | 8 | 34.8 | 2, 8, 12, 14, 20, 22, 23, 26 |
| Acute GVHD | 4 | 15.4 | 17, 18, 22, 24 |
| Chronic GVHD | 1 | 3.8 | 21 |
| Graft failure | 3 | 11.5 | 1, 6, 19 |
| . | n . | % . | Patient reference ID . |
|---|---|---|---|
| Sex | |||
| Female | 11 | 42.3 | 1, 5, 7, 8, 11, 12, 20, 21, 22, 23, 25 |
| Male | 15 | 57.7 | 2, 3, 4, 6, 9, 10, 13, 14, 15, 16, 17, 18, 19, 24, 26 |
| UCB graft | |||
| Single unit | 24 | 92.3 | All except 19, 24 |
| Double units | 2 | 7.7 | 19, 24 |
| Hematologic malignancies | |||
| ALL | 11 | 42.3 | 1, 3, 8, 10, 13, 14, 15, 17, 18, 23, 26 |
| MDS | 8 | 30.8 | 5, 6, 7, 9, 12, 21, 22, 24 |
| AML | 4 | 15.4 | 2, 4, 20, 25 |
| Others | |||
| FHLH | 1 | 3.8 | 11 |
| Farber disease | 1 | 3.8 | 16 |
| Hurler syndrome | 1 | 3.8 | 19 |
| HLA disparity | |||
| 0 | 9 | 34.6 | 1, 4, 5, 6, 12, 14, 19, 23, 25 |
| 1 | 9 | 34.6 | 2, 3, 8, 10, 11, 16, 20, 21, 26 |
| 2 | 8 | 30.8 | 7, 9, 13, 15, 17, 18, 22, 24 |
| Outcome | |||
| Death | 8 | 30.8 | 1, 2, 7, 8, 12, 14, 19, 23 |
| Cytomegalovirus infection | 3 | 11.5 | 7, 9, 12 |
| Herpes virus infection (other) | 9 | 34.6 | 2, 9, 10, 11, 13, 15, 17, 21, 25 |
| Leukemic relapse | 8 | 34.8 | 2, 8, 12, 14, 20, 22, 23, 26 |
| Acute GVHD | 4 | 15.4 | 17, 18, 22, 24 |
| Chronic GVHD | 1 | 3.8 | 21 |
| Graft failure | 3 | 11.5 | 1, 6, 19 |
Conditioning regimen and GVHD prophylaxis are as described in “Methods.” Twenty-three (88.5%) of 26 subjects underwent transplantations to treat persistent malignant disease. The primary cause of posttransplantation mortality was leukemic relapse (n = 5). The frequency of leukemic relapse was calculated among subjects who had hematologic malignancies (n = 23). Subjects lost to follow-up (n = 2) were not included in the analysis.
ALL indicates acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; AML, acute myeloid leukemia; and FHLH, familial hemophagocytic lymphohistiocytosis.
Generation of T-cell microcultures
Frozen UCBMCs and PBMCs were thawed and cultured for 24 hours in RPMI 1640 supplemented with 20% vol/vol FBS. Cells were seeded at 2000 cells per well in 96-well round-bottom plates and were cocultured with 8 × 104 irradiated (300 Gy) 221.A2 cells (EBV-transformed B lymphoblastoid cell line expressing HLA-A2*020120 pulsed with the HLA-A2–restricted modified Melan-A26-35 A27L peptide; ELAGIGILTV; New England Peptide). Alternatively, to achieve polyclonal expansion for repertoire analysis, cells were pulsed with 1 μg/mL PHA (Sigma-Aldrich). T-cell microcultures were maintained in RPMI 1640 supplemented with 10% vol/vol FBS, 50 IU/mL recombinant human (rh) IL-2 (Hoffmann-La Roche), and 10 ng/mL rhIL-7 (R&D Systems). Medium was replenished twice a week.
Isolation and immunophenotyping of Melan-A–specific T cells
To detect Melan-A–specific CD8+ T cells in UCBMC, PBMC, and T-cell microcultures, biotinylated HLA-A2 monomers were refolded around the Melan-A26-35 A27L peptide (CANVAC Core Facility, Montreal, QC) and were tetramerized with PE-conjugated ExtrAvidin (Sigma-Aldrich) or allophycocyanin (APC)–conjugated streptavidin (BD Biosciences). Cells were incubated for 15 minutes at 37°C with 0.3 μg of peptide-MHC tetramers per 1 × 106 cells. mAbs for cell-surface staining were then added, and cells were incubated for 30 minutes at room temperature. Tetramer-specific CD8+ cells were sorted at low pressure in 100% vol/vol FBS with a FACSVantage SE equipped with CellQuest software (BD Biosciences). Compensation and logical gates were set with single fluorochromes. Lymphocytes were gated according to forward and side scatter. Melan-A–specific T cells were gated based on expression of CD8. Samples were defined as tetramer positive when (1) A2/Melan-A+ CD8+ T cells represented > 0.01% of CD8+ T cells, because this frequency corresponded to maximum background of negative controls, and (2) mean fluorescence intensity between tetramer-positive and -negative populations was > 1 log10 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Analysis of cell-surface markers was performed with FCS Express version 3 software (De Novo Software). Tetramer-sorted T cells were directly seeded at 10-50 cells per well in 96-well round-bottom plates and were cocultured with 8 × 104 irradiated (300 Gy) 221.A2 cells per well in RPMI 1640 supplemented with 50% vol/vol FBS, 50 IU/mL rhIL-2, 10 ng/mL rhIL-7, and 1 μg/mL Melan-A26-35 A27L peptide. Medium was changed every 2 to 3 days, and 2 × 104 irradiated 221.A2 cells per well were added once a week. The functionality of Melan-A sorted T cells was tested after 2 weeks in culture (see below). Immunophenotyping was performed with the following mAbs: PE- or APC-conjugated anti-CD8 (RPA-T8), PE-Cy7–conjugated anti-CCR7 (3D12), and FITC-conjugated anti-CD45RA (HI100; BD Biosciences). CD8+ T cells were defined as naive (CD45RA+CCR7+), central memory (CD45RA−CCR7+), effector memory (EM; CD45RA−CCR7−), and terminally differentiated effectors (EMRAs; CD45RA+CCR7−).14 Separately, PBMCs were stained with FITC- or PE-conjugated anti-PD-1 (MIH4) and APC-conjugated anti-CD8 (RPA-T8; BD Biosciences).
Intracellular staining
Tetramer-sorted microcultures (2 × 105 cells) were incubated with APC-conjugated anti-CD107a mAb (H4A3; BD Biosciences) and 221.A2 cells in the presence of 10 μg/mL Melan-A26-35 A27L peptide at 37°C in RPMI 1640 medium supplemented with 10% vol/vol FBS. Culture medium alone was used as negative control. After 1-hour stimulation, 10 μg/mL brefeldin A and 6 μg/mL monensin (BD Biosciences) were added. Cells were incubated for 6 hours, washed with FACS buffer, stained with PerCP-Cy5.5–conjugated anti-CD8 mAb (RPA-T8; BD Biosciences), and fixed and permeabilized with Cytofix/Cytoperm reagents (BD Biosciences). Cells were then stained with PE-conjugated anti–IFN-γ (4S.B3) and FITC-conjugated anti–IL-2 (MQ1-17H12) mAbs (BD Biosciences) for 30 minutes at 4°C and washed twice in Perm/Wash buffer (BD Biosciences). Viable CD8+ T cells were gated for analysis. Background frequencies measured in the absence of cognate peptide were subtracted from each subpopulation.
Cytotoxicity testing
Cytolytic activity of tetramer-sorted, Melan-A–stimulated T-cell microcultures was measured with a conventional 4-hour 51Cr release assay.21 51Cr-labeled targets were 221.A2 cells pulsed with Melan-A26-35 A27L. The effector-to-target ratio was 10:1 (2 × 103 target cells per well). Each microculture was tested in duplicate with Melan-A26-35 A27L peptide and 221.A2 cells alone.
Amplification and sequencing of TCR β-chain transcripts
Total mRNA was extracted from pellets of tetramer-sorted cells (500-2000 cells) with the PicoPure system (Molecular Devices). TCR β-chain V region mRNA, including its third complementarity-determining region (CDR3), was reverse transcribed, and resulting cDNA was amplified by PCR (40 cycles) with universal primers, as described previously.22 PCR products were subcloned into the TOPO TA cloning vector (Invitrogen) and sequenced unidirectionally with an ABI 3730xl automated sequencer (Applied Biosystems; Plateforme de séquençage et de génotypage des genomes, Centre de recherche du CHUL, Quebec, QC). Identification of TCR β-chain V region and TCR β-chain J region segments and determination of CDR3 length and amino acid sequences were performed with IMGT/V-QUEST and confirmed manually.23 Clonal diversity and dominance were estimated with Simpson's diversity index (Ds).24
Statistical analysis
Normality of data distribution was tested with the D'Agostino and Pearson omnibus normality test and the Kolmogorov-Smirnov test. Data were expressed as the mean and standard error or median and interquartile range. Where appropriate, statistical significance in between-groups comparisons was assessed with the Mann-Whitney U test, the Wilcoxon signed rank test, or 1-way ANOVA and Bonferroni multiple comparison test. Relationships between variables were tested with Spearman rank correlation test. P values < .05 were considered statistically significant. All analyses were performed with GraphPad Prism Version 4 (GraphPad Software).
Results
Ex vivo detection of Melan-A–specific T cells in UCB and UCBT recipients
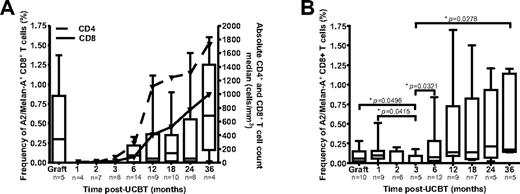
Frequencies of A2/Melan-A+ CD8+ T cells were assessed in UCB grafts and in PBMC samples obtained at 1 to 36 months after UCBT with peptide-MHC tetramers (Figure 1A). A median frequency of 0.3% of A2/Melan-A+ CD8+ T cells was detected in UCB grafts. No A2/Melan-A+ CD8+ T cells were detected at 1, 2, or 3 months after UCBT, consistent with low numbers of CD4+ and CD8+ T cells (Figure 1A). A2/Melan-A+ CD8+ T cells were then detected from 6 to 36 months after UCBT. Relative frequencies of A2/Melan-A+ CD8+ T cells were correlated with total CD4+ and CD8+ T-cell counts in recipients over the entire post-UCBT period (P = .0005 and P = .0008, respectively). This indicates that the relative abundance of antigen-specific CD8+ T cells depends on the reconstitution of the CD4+ and CD8+ T-cell compartments.
Reconstitution of A2/Melan-A+ CD8+ T cells after UCBT. The frequency of A2/Melan-A+ CD8+ T cells was measured ex vivo (A) and after PHA stimulation (B) in UCB graft inoculums and UCBT recipients. Statistical significance was tested with the Mann-Whitney U test. Boxes represent median and interquartile range; error bars represent the range; and n represents the number of subjects.
Reconstitution of A2/Melan-A+ CD8+ T cells after UCBT. The frequency of A2/Melan-A+ CD8+ T cells was measured ex vivo (A) and after PHA stimulation (B) in UCB graft inoculums and UCBT recipients. Statistical significance was tested with the Mann-Whitney U test. Boxes represent median and interquartile range; error bars represent the range; and n represents the number of subjects.
Detection of Melan-A–specific T cells after UCBT after polyclonal stimulation
The early post-UCBT period is characterized by very low numbers of circulating CD8+ T cells.25 To circumvent this limitation, PHA stimulation was used to expand T cells independent of antigenic specificity.26 After 7-10 days of expansion, A2/Melan-A+ CD8+ T cells were detected at a frequency > 0.01% in 9 of 10 grafts and 8 of 9 study subjects at 1 month after UCBT (Figure 1B). These cells were also detected in 2 of 6 and 1 of 5 patients at 2 and 3 months after UCBT, respectively, and in the majority of subjects at 6 to 36 months after UCBT (Figure 1B). The frequency of A2/Melan-A+ CD8+ T cells at 3 months after UCBT was significantly lower than those observed in graft inoculums and in subjects at 1, 6, and 36 months after UCBT (P < .05). Moreover, A2/Melan-A+ T-cell frequencies in the graft inoculum and at 1 month after UCBT were correlated (P = .0252). These results suggest that some of the A2/Melan-A–specific CD8+ T cells present in the graft inoculum persisted in the recipient during the early post-UCBT period and then progressively disappeared.
Evolution of T-cell repertoire diversity after UCBT
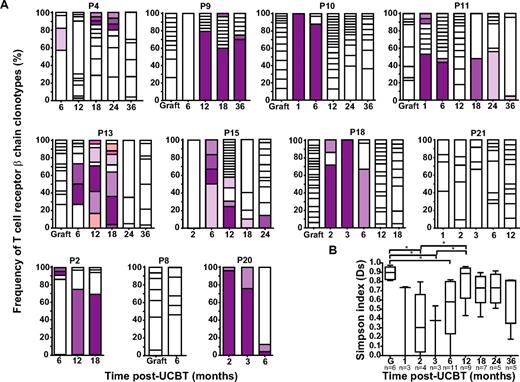
TCR β-chains expressed by A2/Melan-A+ CD8+ T cells were amplified, subcloned, and sequenced. As reported previously,15 A2/Melan-A+ T cells in the graft inoculum were polyclonal (median Ds = 0.90; Figure 2A-B; supplemental Figure 2). Diversity declined significantly at 2 and 3 months after UCBT (P < .05) to increase again at 6 months, albeit to a level significantly lower than that observed in the graft (P < .05). TCR β-chain diversity reached graft-comparable levels at 12 months after UCBT (Figure 2B). Changes in diversity were also reflected at the level of CDR3 length: It was normally distributed in 6 of 6 graft inoculum but became highly biased at 1 to 3 months after UCBT, consistent with repertoire oligoclonality (supplemental Figure 2). From then on, the distribution of CDR3 length progressively normalized, with 2 (18.2%) of 11 subjects exhibiting Gaussian profiles at 6 months, 4 (44.4%) of 9 at 12 months, 3 (42.9%) of 7 at 18 months, and 3 (60.0%) of 5 at 24 months after UCBT (supplemental Figure 2). No common TCR β-chain clonotypes were identified between graft inoculums and UCBT recipients. In contrast, 2 waves of persistent clonotypes were observed in the post-UCBT period. The first wave (“early clonotypes”) emerged between 1 and 3 months and was detected until 6 months after UCBT, whereas the second wave (“late clonotypes”) started to emerge at 6 to 12 months and persisted between 6 and 36 months after UCBT (Figure 2A). It is unlikely that early clonotypes were of recipient origin, because 100% donor chimerism was observed at 3 and 6 months after UCBT in most study subjects. These results are consistent with rare UCB-derived T cells transiently persisting in the recipient up to 6 months after UCBT, followed by progressive repletion of T-cell repertoire diversity via thymopoiesis.
Evolution of the TCR β-chain repertoire in A2/Melan-A+ CD8+ T cells. (A) Longitudinal analysis of the distribution of TCR β-chain clonotypes amplified from graft inoculums and PBMC samples obtained from corresponding UCBT recipients (n = 11). Each bar represents a time point (ie, graft inoculum or time after UCBT), and each box within a bar represents a particular T-cell clonotype identified by a characteristic TCR β-chain CDR3 sequence. Open boxes represent unique clonotypes (ie, clonotypes that were not found at another time point). Colored bars represent persisting clonotypes, that is, clonotypes that were observed at more than 1 time point; identical colors correspond to identical clonotypes in within-patient but not between-patient analysis. Twenty to 35 independent recombinant clones were analyzed per time point. Patients 2, 8, and 20 (P2, P8, and P20, respectively) experienced leukemic relapse. (B) Simpson's diversity index (Ds)24 was used to represent clonal diversity. Statistical significance was tested with 1-way ANOVA and Bonferroni multiple comparison test. *P < .05. Boxes represent median and interquartile range; error bars represent the range; and n represents the number of subjects.
Evolution of the TCR β-chain repertoire in A2/Melan-A+ CD8+ T cells. (A) Longitudinal analysis of the distribution of TCR β-chain clonotypes amplified from graft inoculums and PBMC samples obtained from corresponding UCBT recipients (n = 11). Each bar represents a time point (ie, graft inoculum or time after UCBT), and each box within a bar represents a particular T-cell clonotype identified by a characteristic TCR β-chain CDR3 sequence. Open boxes represent unique clonotypes (ie, clonotypes that were not found at another time point). Colored bars represent persisting clonotypes, that is, clonotypes that were observed at more than 1 time point; identical colors correspond to identical clonotypes in within-patient but not between-patient analysis. Twenty to 35 independent recombinant clones were analyzed per time point. Patients 2, 8, and 20 (P2, P8, and P20, respectively) experienced leukemic relapse. (B) Simpson's diversity index (Ds)24 was used to represent clonal diversity. Statistical significance was tested with 1-way ANOVA and Bonferroni multiple comparison test. *P < .05. Boxes represent median and interquartile range; error bars represent the range; and n represents the number of subjects.
Phenotypic reconstitution of total and A2/Melan-A+ CD8+ T cells after UCBT
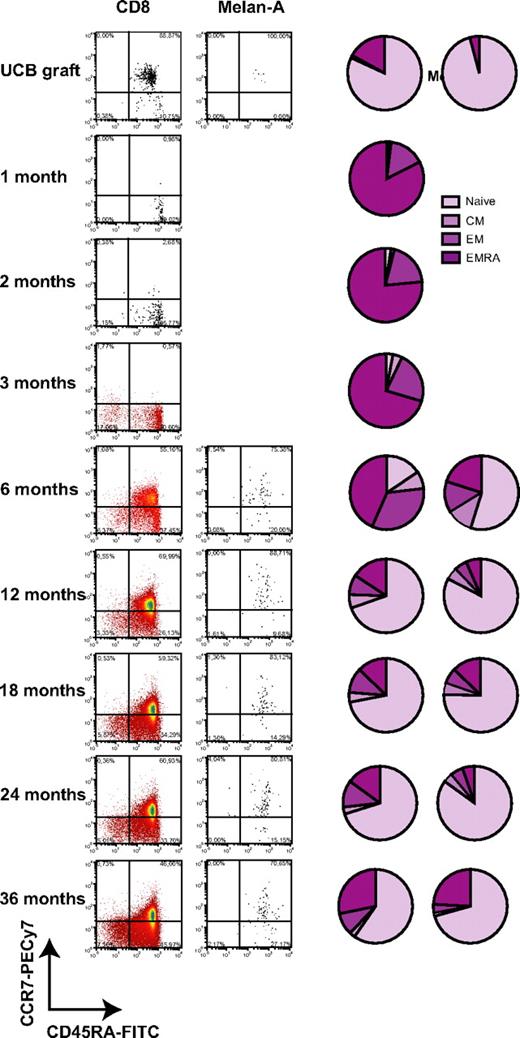
Expression of CD45RA and CCR7 was monitored in total CD8+ T cells and A2/Melan-A+ CD8+ T cells during the post-UCBT period (Figure 3). Total and A2/Melan-A+ CD8+ T cells were mostly naive in graft inoculums (Figure 3; supplemental Figure 3). Low frequencies of naive T cells (1.2%, 2.5%, and 3.0%) and high frequencies of EMRAs (82.5%, 76.5%, and 70.4%) were found in total CD8+ T cells at 1, 2, and 3 months after UCBT (Figure 3). CD8+ T cells were also composed of 15.6%, 19.5%, and 22.5% EMs and 0.7%, 1.5%, and 4.1% central memory cells at 1, 2, and 3 months after UCBT. At 6 months, naive T cells emerged at a median frequency of 15.6% of total CD8+ T cells, whereas 43.4% continued to exhibit an EMRA phenotype. At that time, A2/Melan-A+ CD8+ T cells were detected, and a median of 54.8% of them were naive. From 12 to 36 months after UCBT, the vast majority of CD8+ T cells were naïve, and the second population in terms of relative abundance were EMRAs. At all time points, the proportion of naive cells was significantly higher in A2/Melan-A+ CD8+ T cells than in total CD8+ T cells (P < .0313; Figure 3 and data not shown). Interestingly, naive CD8+ T cells were observed at a frequency of 22.6% at 1 month after UCBT in subject P11, a 5-month-old patient who had already achieved a high TCR repertoire diversity at 1 month after UCBT (Figure 2A). These results indicate that the vast majority of CD8+ T cells display a terminally differentiated phenotype in the first 6 months after UCBT.
Phenotypic reconstitution of total CD8+ T cells and A2/Melan-A+ CD8+ T cells after UCBT. Representative dot plots and pie charts represent median frequencies of naive, central memory (CM), EM, and EMRA CD8+ T cells measured ex vivo in the graft inoculum and UCBT recipients with tetramer staining. No A2/Melan-A+ T cells were detected ex vivo at 1 to 3 months after UCBT.
Phenotypic reconstitution of total CD8+ T cells and A2/Melan-A+ CD8+ T cells after UCBT. Representative dot plots and pie charts represent median frequencies of naive, central memory (CM), EM, and EMRA CD8+ T cells measured ex vivo in the graft inoculum and UCBT recipients with tetramer staining. No A2/Melan-A+ T cells were detected ex vivo at 1 to 3 months after UCBT.
Functional reconstitution of A2/Melan-A+ CD8+ T cells after UCBT
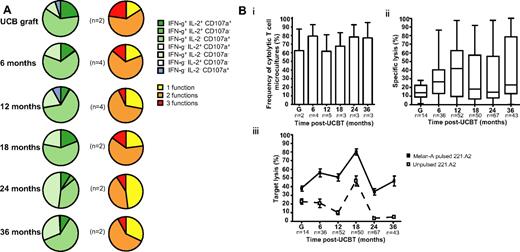
Polyfunctionality is the hallmark of protective T-cell responses against viruses and cancer.27 PBMCs from UCBT recipients were expanded in vitro for 7-10 days in the presence of IL-2, IL-7, and Melan-A26-35 A27L peptide, sorted with A2/Melan-A tetramers, and used in functional assays. After stimulation with cognate peptide, no significant differences in expression of IFN-γ, IL-2, CD107a (monofunctional, bifunctional, polyfunctional), or cytolytic activity were observed in A2/Melan-A+ CD8+ T cells between graft inoculums and samples obtained at 6 to 36 months after UCBT (P > .05; Figure 4; supplemental Figure 4). A2/Melan-A+ CD8+ T cells were not retrieved in sufficient numbers to perform these analyses at 1 to 3 months after UCBT. These results suggest that CD8+ T cells isolated from UCB were capable of expressing combinations of differentiated functions in response to stimulation with cognate peptide, as were those isolated from the peripheral blood of UCBT recipients at 6 to 18 months after transplantation.
Polyfunctionality profile and cytolytic activity of A2/Melan-A+ CD8+ T cells after UCBT. (A) Staining for IFN-γ, IL-2, and CD107a was performed on T-cell microcultures derived from graft inoculums and UCBT recipients after stimulation with cognate peptide. Background frequencies measured in the absence of cognate peptide were subtracted. Left panel represents the frequencies of cells expressing combinations of IFN-γ, IL-2, and CD107a. Right panel depicts the frequencies of cells exhibiting 1, 2, and 3 functions. n represents the number of subjects. (B) 51Cr release assays were performed with 221.A2 targets pulsed with Melan-A26-35 A27L peptide. Samples were considered positive when 51Cr release measured in the presence of cognate peptide was ≥ 10% of total release and ≥ 2 standard deviations above 51Cr release measured in the absence of peptide. (i) Bars represent the mean and standard error of frequencies of cytolytic A2/Melan-A+ CD8+ T-cell microcultures derived from the graft inoculum (G) and from samples obtained from study subjects after UCBT. n represents the number of subjects tested at each time point. (ii) Box-and-whisker plots represent percentage of specific lysis exhibited by T-cell microcultures derived from the graft inoculums or from samples obtained from UCBT recipients. Percentage of specific lysis was computed as (51Cr release in presence of cognate peptide − 51Cr release in absence of peptide) ×100/total 51Cr release. n represents the number of T-cell microcultures tested at each time point. (iii) Squares represent the mean and standard error of lysis in the presence (squf) or absence (squo) of cognate peptide. n represents the number of T-cell microcultures tested at each time point. There were no significant differences between time points. Statistical significance was tested with ANOVA or the Mann-Whitney U test.
Polyfunctionality profile and cytolytic activity of A2/Melan-A+ CD8+ T cells after UCBT. (A) Staining for IFN-γ, IL-2, and CD107a was performed on T-cell microcultures derived from graft inoculums and UCBT recipients after stimulation with cognate peptide. Background frequencies measured in the absence of cognate peptide were subtracted. Left panel represents the frequencies of cells expressing combinations of IFN-γ, IL-2, and CD107a. Right panel depicts the frequencies of cells exhibiting 1, 2, and 3 functions. n represents the number of subjects. (B) 51Cr release assays were performed with 221.A2 targets pulsed with Melan-A26-35 A27L peptide. Samples were considered positive when 51Cr release measured in the presence of cognate peptide was ≥ 10% of total release and ≥ 2 standard deviations above 51Cr release measured in the absence of peptide. (i) Bars represent the mean and standard error of frequencies of cytolytic A2/Melan-A+ CD8+ T-cell microcultures derived from the graft inoculum (G) and from samples obtained from study subjects after UCBT. n represents the number of subjects tested at each time point. (ii) Box-and-whisker plots represent percentage of specific lysis exhibited by T-cell microcultures derived from the graft inoculums or from samples obtained from UCBT recipients. Percentage of specific lysis was computed as (51Cr release in presence of cognate peptide − 51Cr release in absence of peptide) ×100/total 51Cr release. n represents the number of T-cell microcultures tested at each time point. (iii) Squares represent the mean and standard error of lysis in the presence (squf) or absence (squo) of cognate peptide. n represents the number of T-cell microcultures tested at each time point. There were no significant differences between time points. Statistical significance was tested with ANOVA or the Mann-Whitney U test.
Evolution of PD-1+ T-cell frequency during the post-UCBT period
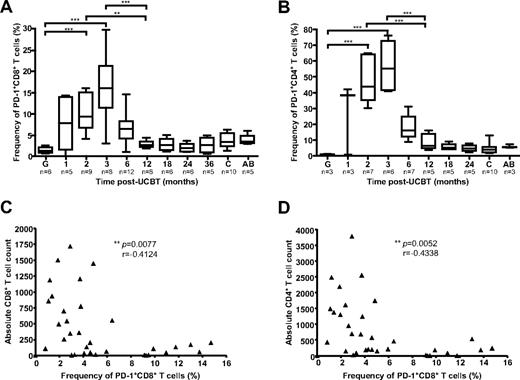
PD-1, an inhibitory receptor of the CD28 family, is highly expressed on dysfunctional CD8+ and CD4+ T cells during chronic viral infections.28 Because the first 3 months after UCBT are associated with a high risk of OI,5 with functional immunodeficiency, and with terminally differentiated CD8+ T cell phenotypes (Figure 3), frequencies of PD-1+ CD4+ and PD-1+ CD8+ T cells were assessed in 18 study subjects, 10 healthy children (3-36 months old), and 5 healthy adults (Figure 5). PD-1 was expressed at very low levels in UCB grafts (1.3% and 1.0% of CD8+ and CD4+ T cells, respectively). Major changes were observed in the mean frequency of PD-1+ T cells during the posttransplantation period (P < .0001). These disparities were accounted for by a remarkable increase in frequency of PD-1+ CD8+ and PD-1+ CD4+ T cells at 2 and 3 months after UCBT compared with graft inoculums and with samples obtained at 6-36 months after UCBT (P < .05; Figure 5A-B; supplemental Figure 5A). The frequencies of PD-1–expressing CD8+ T cells and CD4+ T cells at 3 months after UCBT were also significantly higher than those observed in healthy adults and healthy children (P < .0001), consistent with recent reports.29,30 Frequencies of PD-1+ CD8+ T cells were inversely correlated with CD8+ and CD4+ T-cell counts (P = .0077, r = −0.4124, and P = .0052, r = −0.4338, respectively), which highlights the association between lymphopenia and impaired CD8+ T-cell function in the first months after UCBT (Figure 5C-D). Overall, median frequencies of PD-1+ T cells were higher in the CD4+ than in the CD8+ T-cell compartment (P = .0039), and the frequency of PD-1+ CD8+ T cells was correlated with the frequency of PD-1+ CD4+ T cells throughout the follow-up period (P < .0001, r = 0.7665; supplemental Figure 5B-C). Finally, the normalized median fluorescence index of PD-1 in CD8+ T cells and CD4+ T cells was not higher in the first months after UCBT than later during follow-up (P > .05; supplemental Figure 5D-E). These results are consistent with UCB CD8+ T cells undergoing massive oligoclonal expansion while acquiring a skewed exhausted phenotype after transfer into the recipients.
Frequencies of PD-1+ CD8+ and PD-1+ CD4+ T cells after UCBT. (A) PD-1 expression was analyzed by FACS on CD8+ T cells ex vivo. Gates were set on CD8hi to exclude CD8+ natural killer cells. (B) PD-1 expression was analyzed by FACS on CD4+ T cells ex vivo. (C) Correlation between the frequency of PD-1+ CD8+ T cells and absolute CD8+ T-cell counts throughout the follow-up period. (D) Correlation between the frequency of PD-1+ CD8+ T cells and absolute CD4+ T-cell counts throughout the follow-up period. Box-and-whisker plots depict the median, interquartile range, and range of frequencies in graft inoculums (G), UCBT recipients, healthy children (c), and healthy adults (AB). Statistical significance was tested with Spearman rank correlation test or 1-way ANOVA and Bonferroni multiple comparison test. *P < .05; **P < .01; ***P < .001. n represents the number of subjects.
Frequencies of PD-1+ CD8+ and PD-1+ CD4+ T cells after UCBT. (A) PD-1 expression was analyzed by FACS on CD8+ T cells ex vivo. Gates were set on CD8hi to exclude CD8+ natural killer cells. (B) PD-1 expression was analyzed by FACS on CD4+ T cells ex vivo. (C) Correlation between the frequency of PD-1+ CD8+ T cells and absolute CD8+ T-cell counts throughout the follow-up period. (D) Correlation between the frequency of PD-1+ CD8+ T cells and absolute CD4+ T-cell counts throughout the follow-up period. Box-and-whisker plots depict the median, interquartile range, and range of frequencies in graft inoculums (G), UCBT recipients, healthy children (c), and healthy adults (AB). Statistical significance was tested with Spearman rank correlation test or 1-way ANOVA and Bonferroni multiple comparison test. *P < .05; **P < .01; ***P < .001. n represents the number of subjects.
PD-1 expression is associated with leukemic relapse
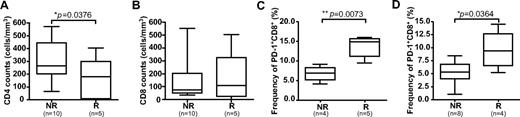
Eight study subjects experienced leukemic relapse during follow-up (“relapsers”), and 6 of these subjects relapsed > 6 months after UCBT (supplemental Figure 3). There were no significant differences in the naive-memory phenotype of CD8+ T cells at 2 or 3 months after UCBT in relapsers compared with nonrelapsers (supplemental Figure 3). However, there was a trend toward higher frequencies of EM T cells (P = .0823) and significantly lower CD4+ T-cells counts in relapsers at 6 months (P = .0376; Figure 6A; supplemental Figure 6). There was no significant difference in total CD8+ T-cell counts between relapsers and nonrelapsers (Figure 6B). Finally, the frequency of PD1+ CD8+ T cells was significantly higher in relapsers than in nonrelapsers at 2 and 6 months (P = .0079 and P = .0364, respectively; Figure 6C-D; supplemental Figure 5G). The frequency of PD-1+ CD4+ T cells was also higher in relapsers at 6 months, but this difference was not statistically significant (supplemental Figure 5F,H). Taken together, these results suggest that a high frequency of PD-1+ CD8+ T cells in UCBT recipients during the early posttransplantation period could be associated with subsequent leukemic relapse.
Association between PD-1 expression and leukemic relapse. Absolute CD4+ T-cell counts (A) and absolute CD8+ T-cell counts (B) were measured by FACS in PBMC samples obtained at 6 months after UCBT. Frequencies of PD-1+ CD8+ T cells were measured in PBMC samples obtained at 2 months (C) and 6 months (D) after UCBT. Statistical significance was tested with the Mann-Whitney U test. Box-and-whisker plots depict the median, interquartile range, and range. n represents the number of subjects. NR indicates nonrelapsers; and R, relapsers.
Association between PD-1 expression and leukemic relapse. Absolute CD4+ T-cell counts (A) and absolute CD8+ T-cell counts (B) were measured by FACS in PBMC samples obtained at 6 months after UCBT. Frequencies of PD-1+ CD8+ T cells were measured in PBMC samples obtained at 2 months (C) and 6 months (D) after UCBT. Statistical significance was tested with the Mann-Whitney U test. Box-and-whisker plots depict the median, interquartile range, and range. n represents the number of subjects. NR indicates nonrelapsers; and R, relapsers.
Discussion
UCB is increasingly being used as an alternative source of hematopoietic stem cells for the treatment of various blood disorders in cases in which a suitably matched donor is not readily available. However, clinical outcomes associated with UCBT differ from those observed after BMT, particularly in terms of GVHD, graft failure, and OI. Leukemic relapse can occur after UCBT, particularly in patients with poor immune reconstitution.31 To explore the cellular and molecular basis of these outcomes, we examined the reconstitution of antigen-specific T lymphocytes in a group of pediatric UCBT recipients. In recent years, several groups have examined the reconstitution of T cells specific for CMV32 or for leukemia-associated antigens such as PR133 and WT1.34 In contrast, the present study was largely focused on the repertoire of T cells that recognize the Melan-A26-35 A27L peptide in the context of HLA-A2 (A2/Melan-A), because these cells represent one of the only preimmune T-cell repertoires that can be studied in humans, particularly when the number of cells that can be obtained from study subjects is limited.15,17,18 Consistent with these previous reports, clonally diversified A2/Melan-A+ CD8+ T cells were reproducibly detected in UCB graft inoculums. Using peptide-MHC tetramers, these cells were not detectable ex vivo in the peripheral blood of UCBT recipients at 1, 2, or 3 months after UCBT, an important “window of susceptibility” to OI. When PHA stimulation was used to compensate for low numbers of total CD8+ T cells in the early posttransplantation period, A2/Melan-A+ CD8+ T cells were revealed in the majority of subjects at 1 month after transplantation and in one third and one fifth of subjects at 2 and 3 months, respectively. These cells exhibited reduced levels of clonal diversity and skewed distribution in CDR3 length compared with graft inoculums and the late posttransplantation period (ie, ≥ 6 months after UCBT), which reflects the oligoclonality of the antigen-specific T-cell compartment. These results are consistent with previous reports on reconstitution of TCR repertoire diversity based on analysis of total CD8+ T cells.35 There were no instances of overlap between the clonotypic profiles observed in the graft inoculum and those observed after UCBT. UCB T cells are highly polyclonal,15 which possibly limits our ability to track clonal persistence between UCB graft inoculums and UCBT recipients. ATG, which was administered to study subjects as part of the conditioning regimen, could also have contributed to the sudden drop in clonal diversity observed after UCBT. In contrast, the temporary persistence of T-cell clonotypes was observed between 1-6 months after UCBT. Chimerism results confirmed that these cells were of donor origin (data not shown). Taken together, these results suggest that the A2/Melan-A–specific T-cell repertoire present in the graft inoculum was not preferentially mobilized after transfer into the UCBT recipient but that some of these cells transiently persisted in UCBT recipients up to 6 months after transplantation, followed by reconstitution of the T-cell repertoire via thymopoiesis. Therefore, the present results are compatible with the existence of a “hole” or “through” in the T-cell repertoire between 2 and 3 months after UCBT, with little overlap between a first wave derived from homeostatic expansion of T cells contained within the UCB graft inoculum and a second wave generated by rising thymic output.
Most A2/Melan-A+ CD8+ T cells and CD8+ T cells present in graft inoculums expressed a CD45RA+ CCR7+ naive cell-surface phenotype. In contrast, the vast majority of CD8+ T cells displayed a terminally differentiated CD45RA+ CCR7− phenotype in the first 6 months after UCBT, consistent with previous in vitro studies showing that UCB T cells differentiated mostly into EMRAs after stimulation and with observations in adult UCBT recipients and children who underwent BMT.15,36,37 The EMRA phenotype has been associated with shorter T cell t1/2 and is concordant with the short-term persistence and progressive disappearance of graft-derived CD8+ T cells during the first 3-6 months after UCBT, followed by the emergence of clonally diversified naive T cells through thymopoiesis. Interestingly, naive CD8+ T cells were observed at a frequency of 22.6% at 1 month after UCBT in subject P11, who exhibited very high TCR β-chain diversity as early as 1 month after UCBT and clonotypic persistence until 18 months. This suggests that naive T cells and the persistent T-cell clonotype observed in subject P11 resulted from de novo generation of thymus-derived T cells rather than homeostatic expansion of graft-derived T cells, compatible with unusually active thymopoietic processes in this 5-month-old patient. Quantification of the levels of TCR rearrangement circles in isolated T-cell subsets and other measurements that could be used to strengthen this hypothesis were not performed in the present study as a result of strict limitations in biologic sample size.38,39
There were no significant differences observed between A2/Melan-A+ CD8+ T cells isolated from the graft inoculums and from UCBT recipients at 6-18 months after transplantation in terms of IFN-γ, IL-2, CD107a secretion, and cytolytic activity after stimulation with cognate peptide. The T-cell polyfunctionality profile is a key indicator of the efficacy of antiviral and antitumoral cell-mediated immune responses.27 Hence, although this profile was not determined at 1-3 months because of limitations in biologic sample size, these results indicate that the functionality of antigen-specific CD8+ T cells was fully reconstituted in UCBT recipients at and beyond 6 months after UCBT. Had larger amounts of cells (> 107) from graft inoculums and recipients been available, it would have been of great interest to compare these results with the evolution of another biologically relevant preimmune repertoire in the context of UCBT, such as the CD8+ T-cell population specific for the A2/CMVpp65 tetramer.18
PD-1, an inhibitory receptor of the CD28 family, is highly expressed on dysfunctional CD8+ and CD4+ T cells during chronic viral infections.28 PD-1 contributes to the dampening of antiviral and antitumoral immunity and is associated with clonal exhaustion and diminished effector functions.40-42 During the early posttransplantation period, the frequency of PD-1+ CD8+ T cells was positively correlated with that of PD1+ CD4+ T cells and inversely correlated with CD8+ and CD4+ T-cell counts, which highlights the association between lymphopenia and impaired CD8+ T-cell function in the first months after UCBT. Gallez-Hawkins et al43 reported no increase in the frequency of PD-1+ CD8+ or CD4+ T cells in the first months after BMT. In the present study, all UCBT recipients experienced viral infections in the first 3 months after transplantation, which makes it impossible to assess the impact of OI on PD-1 expression or the impact of PD-1 expression on susceptibility to viruses. Additional studies will be needed to verify whether these differences are related to (1) the source of the graft inoculum (UCB versus BM), (2) the use of ATG, (3) the cellular dose, (4) the respective ages of the transplant recipients, and/or (5) the differential incidence of GVHD and OI, which could not be assessed in the context of the present study. PD-1 expression is preferentially observed in EM and EMRA T cells.44,45 Overall, the present results are consistent with UCB CD8+ T cells undergoing massive oligoclonal expansion while acquiring a skewed terminally differentiated and exhausted phenotype after transfer into the recipients. These results provide a rationale for the increased susceptibility to OI and comparatively reduced incidence of acute GVHD observed in UCBT recipients.5 These conclusions would be strengthened by a comparison between UCBT and BMT, which should be addressed in future studies. PD-1 expression and/or the interaction between PD-1 and its ligands PD-L1 and PD-L2 could represent attractive targets for interventions that aim to reduce the incidence of OI after UCBT.
Finally, 8 subjects experienced leukemic relapse, including 6 who relapsed later than 6 months after UCBT, a time at which the polyfunctionality profile of antigen-specific CD8+ T cells had already recovered completely. Although the frequency of PD1+ CD8+ T cells in the first 3 months after UCBT was high in every subject, it was significantly higher in relapsers than in nonrelapsers at 2 and 6 months. Lower CD4+ T-cell counts and a higher frequency of EM CD8+ T-cell frequency were also observed in relapsers, which potentially highlights the importance of cooperation between CD4+ and CD8+ T cells for generation, maintenance, and/or efficacy of the graft-versus-leukemia effect. Lower frequencies of CD28− CD8+ and EMRA CD8+ T cells after allogeneic stem cell transplantation previously were associated with a greater risk of relapse of underlying hematologic malignancies.37 To the best of our knowledge, the present study is the first to report an association between the frequency of PD-1+ CD8+ T cells and subsequent leukemic relapse. This should be confirmed in larger series, and additional studies will be required to determine whether increased frequency of PD-1+ CD8+ T cells is the underlying cause or merely a consequence of the ensuing relapse. However, these results suggest that this parameter could be used as a potential prognostic factor for leukemic relapse in pediatric UCBT recipients.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Martine Caty, Serge Sénéchal, and Samira Mezziani for expert technical assistance and Claude Perreault and Françoise Le Deist for critical reading of the manuscript.
This work was supported by grants from le Fonds de la recherche en santé du Québec (FRSQ), Héma-Québec, and Fondation Centre de cancérologie Charles-Bruneau (M.A.C., M.D., H.S.). N.M. was the recipient of scholarships from the Cole Foundation, la Fondation de l'Hôpital Sainte-Justine, and FRSQ.
N.M. is a PhD candidate at Université de Montréal, and this work is submitted in partial fulfillment of the requirement for the PhD.
Authorship
Contribution: N.M. performed research and statistical analysis, collected, analyzed and interpreted data, and wrote the manuscript; M.A.C. and M.D. wrote the manuscript; and H.S. designed research, performed statistical analysis, and wrote the manuscript.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Hugo Soudeyns, Unité d'immunopathologie virale, Centre de recherche du CHU Sainte-Justine, 3175 Côte Sainte-Catherine, Room 6735, Montreal, QC H3T 1C5, Canada; e-mail: hugo.soudeyns@recherche-ste-justine.qc.ca.