The study by Khellaf et al in this issue of Blood suggests that the use of romiplostim outside a formal clinical trial setting can safely produce a sustained clinical benefit in 65% of patients with chronic or refractory primary immune thrombocytopenia (ITP), particularly those who present with less severe bleeding manifestations.1
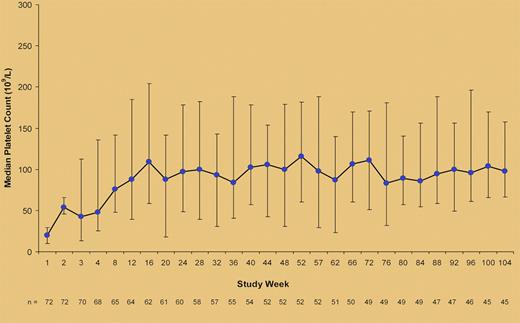
Median (25th-75th percentile) platelet counts during treatment with romiplostim in the study by Khellaf et al.1
Median (25th-75th percentile) platelet counts during treatment with romiplostim in the study by Khellaf et al.1
Splenectomy has historically been the second line therapy for adults with ITP in whom achieving a safe platelet count with initial corticosteroid and/or immunoglobulin therapy has failed. Approximately two-thirds of patients achieve a normal platelet count and others attain a partial response that may allow for a reduction in the use of rescue therapy.2 However, 10% to 15% of patients do not have a clinically significant response and 30% to 35% will eventually experience a relapse (refractory ITP). Furthermore, there is a small risk of perioperative mortality as well as a long-term risk for infection with encapsulated organisms.2 There are no prospectively validated predictors of response to splenectomy, although platelet kinetic studies with 111Indium-labeled autologous platelets appear to be a useful tool in specialized centers.3
Reasons to support a nonsurgical approach as second-line treatment for ITP include the fact that a significant number of remissions can occur up to 3 years from the time of diagnosis4 and the availability of an ever increasing armamentarium of drugs. Besides, there are patients who have medical contraindications to surgery and those who refuse splenectomy.
Therapeutic options available to patients with chronic and refractory ITP have recently expanded with the introduction of 2 thrombopoietin receptor agonists, romiplostim and eltrombopag. Information about the efficacy and safety of these agents derives from clinical trials with selective inclusion criteria. However, evidence of their impact on the management of ITP in clinical practice has been lacking, until now.
Here, Khellaf and colleagues describe the results of 72 patients with chronic ITP treated with romiplostim within a French compassionate-use program since January 2008, before the drug was available on the market.1 Patients' median age was 63 years; one-half had been splenectomized, more than half were receiving treatment for ITP when romiplostim was started, and nearly 20% of them would have not been eligible for clinical trials because of comorbidities such as cardiac arrhythmia, uncontrolled hypertension, or deranged renal function. Romiplostim was started at 1 μg/kg per week and the dose was adjusted to a maximum of 10 μg/kg per week to reach a target platelet-count range of 50-250 × 109/L (see figure). At 2 years, 47 of 72 patients (65%) had achieved a clinical benefit consisting either of achievement of a platelet count ≥ 50 × 109/L and at least double the baseline value (37 patients) or reduction of concomitant ITP medications and/or less severe bleeding manifestations observed with lower increments of the platelet counts (10 patients). Two patients were able to maintain a platelet response when romiplostim was stopped. In multivariate analysis, response to romiplostim was predicted only by the baseline value of the bleeding score that had been previously validated by the same group.5
Romiplostim was generally well tolerated, although 2 patients had to stop treatment because of persistent side effects (headache in 1 patient and arthralgias in the other). The adverse-event profile was similar between patients with and without comorbidities. However, 2 elderly patients with cardiovascular risk factors each experienced a transient ischemic attack without sequelae while their platelet counts were < 100 × 109/L.
The results of this study lend to some considerations. It is now clear that the response rates to romiplostim in the published trials are fairly superimposable to what one can achieve in the clinical arena. There is often the concern that because of selection criteria and stricter control of administration of the drug, results of trials may be better than those observed in real life. However, in the French study, in which patients were unselected and romiplostim was administered at home in 74% of the patients, the overall response rate was nearly as good as that obtained in the pivotal studies.6
That said, there are still roughly one-third of patients for whom the use of romiplostim results in no sustained improvement of the platelet count. In Khellaf et al's study these were the patients presenting with the most severe bleeding manifestations, a finding that is somewhat diminutive of the role of this drug in managing difficult cases. However, this is the first time that a bleeding score in ITP is found to have a predictive value. Because previous trials with either romiplostim or eltrombopag used the World Health Organization bleeding score, this observation needs to be confirmed prospectively.
With regard to side effects of treatment, romiplostim has consistently shown a favorable profile. Nevertheless, because the absolute safety of this drug in the very long term is yet to be defined, its use in young, nonsplenectomized individuals should be carefully pondered. The same concerns, however, also apply to many immunosuppressive agents that need chronic administration. Finally, to minimize the possible increased risk of thromboembolic events,7 a sensible approach is to set the target platelet count range between 50-100 × 109/L. For the majority of patients with a cardiovascular risk this will be a hemostatically safe range even on anticoagulants or antiaggregants.
Despite some of the limitations that we have described, there is little doubt that romiplostim represents a major breakthrough in the management of chronic ITP. In addition to an increase in platelet count, decreased risk of bleeding, and reduction or discontinuation of concomitant ITP medications, patients responding to romiplostim also experience an often dramatic improvement of health-related quality of life.8,9 This aspect could not be adequately addressed in the retrospective French study, but clinicians treating patients with ITP are aware that it is as important as the platelet count.
Future strategies for the development of romiplostim in chronic ITP may involve its use in combination therapies, with the aim of targeting both the increased platelet destruction and the impaired platelet production that is typical of this disease.10
Conflict-of-interest disclosure: The author has served as a consultant for Amgen, GlaxoSmithKline, and Suppremol and has participated on advisory boards and/or as a speaker at medical education events supported by Amgen, GlaxoSmithKline, Nycomed, Novo, Bayer, and Baxter. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal