Abstract
The pathophysiology of iron-induced compromised fertility in women with thalassemia major (TM) was evaluated in 26 adult TM females. Low gonadotropin secretion resulted in reduced ovarian antral follicle count and ovarian volume, but levels of anti-müllerian hormone (AMH), a sensitive marker for ovarian reserve independent of gonadotropin effect, were mostly normal. AMH correlated with non–transferrin-bound iron (NTBI), suggesting a role of labile iron in the pathogenesis of decreased reproductive capacity, possibly occurring in parallel to cardiac iron toxicity, as cardiac iron was associated with the presence of amenorrhea and with NTBI levels. AMH emerges as an important biomarker for assessment of reproductive capacity in TM, demonstrating that fertility is preserved in the majority of those younger than 30 to 35 years. AMH can be useful in future studies aiming at improved chelation for fertility preservation, whereas NTBI and labile plasma iron may be valuable for monitoring iron effect on the reproductive system.
Introduction
Despite progress in chelation regimens, the deleterious effect of excess iron to the reproductive system of women with thalassemia major (TM) is still common.1,2 Iron toxicity to the anterior pituitary results in declining synthesis of lutenizing hormone (LH) and follicle-stimulating hormone (FSH).3 The effect of low gonadotropin secretion on the ovarian oocyte maturation has not been explored, and a possible direct effect of iron, in particular that of non–transferrin-bound iron (NTBI) and its redox active form, labile plasma iron (LPI), on the ovaries is unknown. Some have suggested iron-induced ovarian dysfunction1 ; but with reports of successful ovulation-induction and pregnancies, it has been proposed that ovarian function is preserved even in women with amenorrhea.2
Ovarian antral follicle pool can predict fertility capacity, and reproductive aging is directly related to the decline in this pool. Low ovarian reserve is associated with low chances for spontaneous pregnancy and poor response to hormonal stimulation.4-6 Antral follicle count (AFC), visualized by ultrasound, and anti-müllerian hormone (AMH), which corresponds with AFC, can accurately assess ovarian follicle pool and are used for ovarian reserve testing, identifying women at risk for early ovarian failure.7,8 The usefulness of ovarian reserve testing for the care of thalassemia females has not been evaluated. Reproductive capacity in TM women cannot be well predicted by means of age, menstrual status, or transfusion and chelation parameters.1,9 Yet, as overall survival continues to improve,10,11 attainment of reproductive capacity is crucial for many patients, a concern often brought up to the hematologist.
We evaluated the effect of iron burden on hypogonadism and ovarian reserve in TM women, to better understand the pathophysiology of reduced fertility and evaluate the predictive ability of ovarian reserve testing in this unique patient-population.
Methods
Thalassemia women (≥ 17 years of age) who were treated with regular transfusion therapy for at least 10 years before enrollment in the study were eligible. Data from a multiethnic population of normo-ovulatory women (n = 769)12 were used as the normal comparator. Children's Hospital and Research Center Oakland and University of California at San Francisco Institutional Review Board approvals were obtained, and all the participants provided written informed consent in accordance with the Declaration of Helsinki.
Annual liver iron concentration (LIC) was quantitated by superconducting quantum interference device biosusceptometer system; and cardiac iron concentration by magnetic resonance imaging (1.5 Tesla; Philips Intera). NTBI and LPI were measured at a standardized research laboratory (University College London, London, United Kingdom) and vitamin C in a commercial clinical laboratory. Hormone levels were determined in a standardized research laboratory (CLASS Laboratory, University of Michigan). AFC and ovarian volume measurements were performed all by the same examiner, using a Shimadzu SDU-450XL ultrasound machine, with a vaginal transducer.
Statistical analysis was performed using SAS Version 9.2 software. Bivariate Pearson correlations were computed, and linear regression of age on the AMH values was performed. A significance level of .05 was used for all statistical tests.
Results and discussion
Twenty-six females (transfused for 25.4 ± 7.4 years) were studied. Mean LIC was 15.7 ± 12.9 mg/g dry weight. NTBI (n = 23) was 4.2 ± 2.4μM, and LPI 0.8 ± 1.0μM, correlating with each other (r = 0.66; P = .01). Cardiac T2* iron estimation was 19.4 ± 9.4 ms and inversely correlated with LPI (r = −0.5; P = .04) and with NTBI (r = −0.4; P = .059).
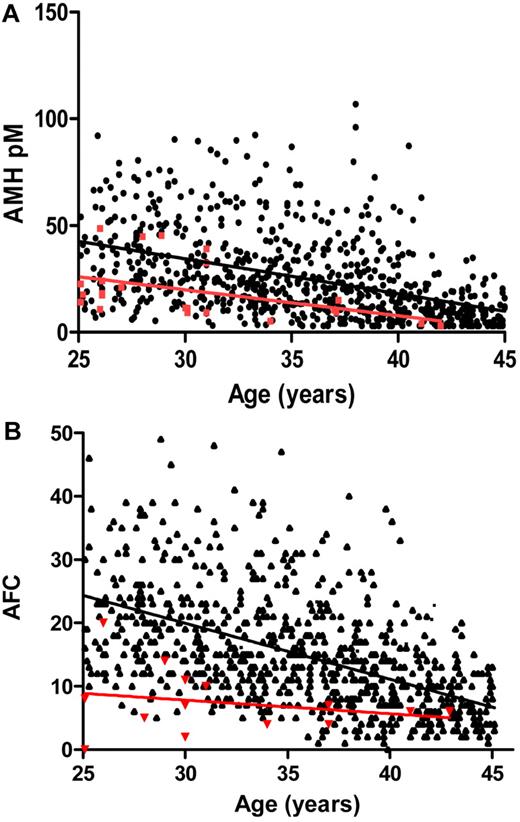
LH and FSH, inhibin B, and estradiol levels were low in 15 to 18 patients (∼ 60%), all amenorrheic (Table 1). AMH inversely correlated with age (r = −0.48, P = .01) and was lower in amenorrheic women. AMH was within the range of the normal controls (20.6 ± 14pM and 25.6 ± 19pM, respectively; 95% confidence interval [CI], 13.4-26.8; Figure 1A). Mean follicle count (AFC) was 7.4 ± 5 (range, 0-20), lower than that in normo-ovulatory women (Figure 1B). Both AMH and AFC were reduced in 5 women older than 30 years (mean, 34 ± 5 years), suggesting combined pituitary and ovarian dysfunction. Ovarian volume was low (1.25 ± 1.2 cm3; range, 0.3-6.8 cm3). FSH, LH, and estradiol levels had no significant correlations with iron overload measures, whereas AMH inversely correlated with NTBI (r = −0.5, P = .01). Mean vitamin C levels were 0.6 ± 0.5 mg/dL (normal, 0.4-2.0 mg/dL) but very low in 11 patients (0.1 ± 0.2 mg/dL), inversely correlating with LIC (r = −0.4, P = .05).
Clinical and endocrine characteristics
| . | Amenorrhea (primary or secondary) . | Normal cycle . | P . |
|---|---|---|---|
| No. (%) of patients | 17 (65) | 9 (35) | — |
| Age, y | 29 ± 5.7 | 27 ± 8 | NS |
| Mean age of secondary amenorrhea, y | 19.2 ± 3 | — | — |
| Years receiving transfusions | 28 ± 6 | 20 ± 6 | .005 |
| LIC, mg Fe/g dry | 13 ± 10 | 17 ± 13 | NS |
| NTBI, μM | 3.7 ± 1.6 | 5.3 ± 3 | NS |
| LH, mIU/mL | 2.7 ± 2.4 | 8 ± 6 | .003 |
| FSH, mIU/mL | 2.2 ± 2.7 | 7.3 ± 9 | .05 |
| E2, pg/mL | 17 ± 22 | 63 ± 60 | .009 |
| Inhibin B, pg/mL | < 10 | 35 ± 15 | .0001 |
| AMH | 15 ± 7.1 | 20.7 ± 14 | NS |
| AFC* | 7.5 ± 5 | 7 ± 1 | NS |
| Mean ovarian volume, cm3* | 1.3 ± 1.4 | 1.3 ± 1 | NS |
| Successful pregnancies | 1 in 1 woman | 5 in 2 women | — |
| Additional endocrinopathies† | 10 (59%) | 2 (22%) | NS |
| Diabetes | 7 | 1 | — |
| Hypothyroidism | 3 | 1 | — |
| Hypoparathyroidism | 0 | 0 | — |
| Cardiac T2* MRI, ms | 16.3 ± 8 (n = 14) | 24.4 ± 10 (n = 8) | .05 |
| . | Amenorrhea (primary or secondary) . | Normal cycle . | P . |
|---|---|---|---|
| No. (%) of patients | 17 (65) | 9 (35) | — |
| Age, y | 29 ± 5.7 | 27 ± 8 | NS |
| Mean age of secondary amenorrhea, y | 19.2 ± 3 | — | — |
| Years receiving transfusions | 28 ± 6 | 20 ± 6 | .005 |
| LIC, mg Fe/g dry | 13 ± 10 | 17 ± 13 | NS |
| NTBI, μM | 3.7 ± 1.6 | 5.3 ± 3 | NS |
| LH, mIU/mL | 2.7 ± 2.4 | 8 ± 6 | .003 |
| FSH, mIU/mL | 2.2 ± 2.7 | 7.3 ± 9 | .05 |
| E2, pg/mL | 17 ± 22 | 63 ± 60 | .009 |
| Inhibin B, pg/mL | < 10 | 35 ± 15 | .0001 |
| AMH | 15 ± 7.1 | 20.7 ± 14 | NS |
| AFC* | 7.5 ± 5 | 7 ± 1 | NS |
| Mean ovarian volume, cm3* | 1.3 ± 1.4 | 1.3 ± 1 | NS |
| Successful pregnancies | 1 in 1 woman | 5 in 2 women | — |
| Additional endocrinopathies† | 10 (59%) | 2 (22%) | NS |
| Diabetes | 7 | 1 | — |
| Hypothyroidism | 3 | 1 | — |
| Hypoparathyroidism | 0 | 0 | — |
| Cardiac T2* MRI, ms | 16.3 ± 8 (n = 14) | 24.4 ± 10 (n = 8) | .05 |
Patients' baseline findings are shown based on the presence or absence of amenorrhea. Hormonal replacement therapy was discontinued for 1 month before obtaining all reproductive measures. Iron chelation was halted until after obtaining NTBI and LPI levels. A factor of 6.0 was applied to convert in vivo LIC (wet weight) into dry weight, comparable to paraffin-embedded biopsies, expressed in mg/g dry weight. Average LIC and results of the measure closest to the time of fertility evaluation were used for analysis. Average previously obtained LIC of entire the group (3.7 ± 2/patient) was 14.5 ± 11 mg/g dry weight.
— indicates not applicable; E2, estradiol; OV, ovarian volume; and NS, not significant.
Follicles with a mean diameter (of 2 dimensions) between 2 and 10 mm were counted. The volume of each ovary was calculated by applying the formula for an ellipsoid: (length × height × width × π/6), the average of both ovaries was then applied.
Other endocrinopathies were noted in 10 women, who had amenorrhea. All 5 patients older than 30 years who had markers of ovarian dysfunction were included in this subgroup.
AMH levels and AFC in TM women and normo-ovulatory controls. (A) AMH levels in the thalassemia women, 25 years and older (n = 23, red circles) were compared with normal controls (●; n = 759), showing that the slopes of the regression lines against age were not statistically different (P = .56). The slope was significant for the normal controls (P < .0001; 95% CI, −1.867 to −1.406) and for the thalassemia patients (P < .03; 95% CI, −2.323 to −0.1142), implying an association with age. There was a 5.0pM (95% CI, 13.4 to 26.8) difference between the group means. The levels in the thalassemia women were in the low-normal range of normal and dropped to lower levels in women older than 30 years. (B) Age-dependent AFC in thalassemia women and normal controls. AFC number includes all counted follicles 2-10 mm in size, in thalassemia women, and in the cohort of normal controls (n = 769).
AMH levels and AFC in TM women and normo-ovulatory controls. (A) AMH levels in the thalassemia women, 25 years and older (n = 23, red circles) were compared with normal controls (●; n = 759), showing that the slopes of the regression lines against age were not statistically different (P = .56). The slope was significant for the normal controls (P < .0001; 95% CI, −1.867 to −1.406) and for the thalassemia patients (P < .03; 95% CI, −2.323 to −0.1142), implying an association with age. There was a 5.0pM (95% CI, 13.4 to 26.8) difference between the group means. The levels in the thalassemia women were in the low-normal range of normal and dropped to lower levels in women older than 30 years. (B) Age-dependent AFC in thalassemia women and normal controls. AFC number includes all counted follicles 2-10 mm in size, in thalassemia women, and in the cohort of normal controls (n = 769).
Our findings imply a decrease in ovarian follicle development, as reflected by low inhibin B, AFC, and ovarian volume, probably because of lack of FSH stimulation. However, AMH levels are overall normal, as they represent early and preantral follicles, which are not affected by the low gonadotropin synthesis. This explains successful cases of induction of follicle growth and ovulation when TM women are stimulated with exogenous gonadotropins. Still, TM women seem to have a premature ovarian aging compared with normo-ovulatory women, mostly in women in their early-mid-30s, raising a concern of the optimal age for best likelihood of successful response to hormonal stimulation. Indeed, case series of successful pregnancy induction report a mean age of 24.5 and 29.5 years.2,13 The rate of decline in ovarian reserve varies considerably between individual women and is probably even more variable among women with TM. Therefore, a test that can provide information regarding the status of a woman's remaining oocytes is of great importance. Recent studies have shown that AMH is the serum marker that best reflects the gradual reduced reproductive capacity with increasing age.14-16 Our results imply that AMH shows significant promise to serve as such a marker in thalassemia women with iron overload and seems a better marker than AFC, which in many cases is lower despite a normal AMH. Thalassemia women had a considerably lower ovarian volume compared with reported normal controls (1.25 ± 1.2 cm3 vs 6-6.6 cm3),17 also representing impaired ovarian reserve, probably a result of a halt in follicle maturation because of lack of gonadotropin stimulation. However, there may also be direct iron toxicity to ovarian tissue.
Ovarian tissue iron overload is supported by the finding of inverse correlation of AMH levels, secreted exclusively by the ovaries, and NTBI. Elevated NTBI, through its labile component, is capable of permeating into organs and compromising organ function.18 An increase in reactive oxygen species and lower enzymatic antioxidant defense mechanisms have been shown to accelerate follicle aging.19 High redox activity in the ovarian follicular fluid of a TM woman was reported, signifying that redox-active iron ions mediate free radical production, inducing ovarian tissue injury.20 A low antioxidant capacity is demonstrated in our patients by having low ascorbic acid levels, which also has a role in oocyte development.21 Further study addressing iron-induced oxidative stress and premature ovarian aging in TM is needed.
The presence of amenorrhea did not correlate with LIC or “free” iron but was associated with higher cardiac iron, other endocrinopathies, and length of time receiving regular transfusions. Interestingly, we found an association of cardiac iron with NTBI and LPI. Lack of correlation of cardiac iron and LIC, but an association with NTBI, was shown before.22 It is probable that labile, potentially toxic, iron plays a major role in causing myocardial as well as reproductive tissue damage,23-25 suggesting a role for “free” iron in the pathogenesis of impaired fertility.
In conclusion, our data suggest that ovarian reserve is preserved in the majority of TM, < 30-35 years old, despite a low follicle count and reduced ovarian volume. AFC is affected by the low gonadotropin secretion and cannot accurately reflect ovarian reserve, making AMH the better marker for determination of reproductive stage. NTBI and LPI may have a significant contribution to the reproductive tissue damage, implying a need to better define the appropriate chelation regimens, the role of antioxidant supplementation, and the markers for improved monitoring of treatment effects on the reproductive capacity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Olivia Vega, Nancy Sweeters, Deborah Trevithick, and Dru Foote for their assistance in coordination of patients' studies and data collection.
This work was supported by the Cooley's Anemia Foundation award on Translational Research in Adult Thalassemia, the National Center for Research Resources (grant UL1RR024131-01), and the National Institutes of Health/National Center for Research Resources (grants R01HD044876 and UCSF-CTSI UL1 RR024131).
This article's contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: S.T.S. designed and performed research, analyzed data, and wrote the manuscript; J.v.D. and M.R. assisted with data analysis; M.I.C. performed all ovarian studies, analyzed data, and assisted in writing the manuscript; E.P.V. assisted in writing and critiquing the manuscript; and G.G. performed statistical analysis and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sylvia T. Singer, Department of Hematology/Oncology and Clinical Research Center Oakland, Children's Hospital and Research Center, 747 52nd St, Oakland, CA 94609; e-mail: tsinger@mail.cho.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal