Abstract
The human plasma protein β2-glycoprotein I (β2-GPI) is the major target of autoantibodies associated with antiphospholipid syndrome. However, the biologic function of this abundant protein is still unclear. Here we identify β2-GPI as a complement regulator. β2-GPI circulates in the plasma in an inactive circular form. On surface binding, such as to apoptotic cells, β2-GPI changes conformation to an elongated form that acquires C3/C3b binding activities. β2-GPI apparently changes conformation of C3, so that the regulator factor H attaches and induces subsequent degradation by the protease factor I. β2-GPI also mediates further cleavage of C3/C3b compared with factor H alone. Our data provide important insights into innate immune regulation by plasma protein β2-GPI, which may be exploited in the prevention and therapy of autoimmune disease antiphospholipid syndrome.
Introduction
β2-glycoprotein I (β2-GPI), also termed apolipoprotein H,1 is a 50-kDa glycosylated human plasma protein with a concentration of 200 μg/mL (4μM)2 that also associates with lipoprotein particles.3 In the autoimmune disease antiphospholipid syndrome (APS), autoantibodies to β2-GPI are identified.4-6 APS is characterized by recurrent vascular thrombosis and pregnancy loss; and in pregnant women, β2-GPI autoantibodies trigger severe complications, resulting in miscarriage, intrauterine growth restriction, and fetal death.7-9 β2-GPI displays anticoagulant activity and inhibits the contact activation of the intrinsic coagulation pathway,10 platelet prothrombinase activity11 and adenosine diphosphate-induced platelet aggregation.12 In addition, β2-GPI also exerts antiangiogenic and antitumor activities.13,14 Despite these activities, the main function of β2-GPI is unknown.
β2-GPI is composed, like complement factor H and factor H-related protein 1, of consecutive short consensus repeat elements (SCRs), also called complement control protein modules, each approximately 60 amino acids in size.15 These repeats are frequently found in proteins with complement-regulatory functions. The fifth domain of β2-GPI has a modified structure, as it contains a 6-residue insertion and an extra 20-amino acid-long mobile tail. Together, these additional amino acids form a binding site for negatively charged, anionic phospholipids, such as phosphatidylserine or cardiolipin,16,17 which are exposed on apoptotic or necrotic cells. Recently, Agar et al18 identified 2 conformations of β2-GPI: a circular inactive form of β2-GPI in the plasma and an elongated, active one when β2-GPI is bound to surfaces. The circular form results from internal interaction of the N-terminal SCRI with the C-terminal SCRV of β2-GPI. This interaction is disrupted by binding of β2-GPI to surfaces, which generates the elongated conformation.18 The crystal structure of β2-GPI shows the open, J-shaped form of β2-GPI.19,20
As the β2-GPI protein function is unclear, we aimed to identify the role of this human plasma protein. Given the structural similarity between β2-GPI and complement factor H and factor H-related protein 1, we hypothesized that β2-GPI harbors complement-regulatory activities. The complement system is a major part of the innate immune system and also directs clearance of dead cells and debris from the body. In addition, complement also activates the adaptive immune response21 and interacts with the coagulation system.22 The complement system is activated via 3 separate pathways (alternative, classic, and lectin) that differ in their mode of recognition but converge in the generation of C3 and C5 convertases.21,23 The C3 convertase of the alternative pathway is formed spontaneously by hydrolysis of C3 to C3(H2O) that can form a C3 convertase, which can amplify autocatalytically. The generated C3 convertase cleaves further C3 molecules to C3b and to the anaphylatoxin C3a. C3b attaches covalently to nearby surfaces, which leads to opsonization and marks the surface for phagocytosis. In addition, the C5 convertase is formed from the C3 convertase by binding an additional C3b molecule. This convertase cleaves C5 and initiates the terminal complement pathway as well as inflammatory reactions.21,23 Complement activation is favored on foreign surfaces, whereas self-surfaces and membranes are protected by membrane-bound and soluble regulators.21,23 In situations of uncontrolled complement activation, the newly formed complement products can harm the endothelium and tissues of the body.24
Apoptotic cells and particles provide surfaces for enhanced C3b deposition.25,26 C3b is then rapidly inactivated to iC3b by recruited soluble regulators, such as factor H, and further complement activation is inhibited.24,26 iC3b generated on the apoptotic surface is recognized by complement receptors CR3 and CR4 on macrophages, which eliminate the apoptotic cells by phagocytosis. Thus, the noninflammatory clearance of apoptotic cells and particles depends in great part on the controlled activity of the complement system. Defects in the clearance of modified or dead cells can lead to the generation of autoantibodies and autoimmune diseases, such as systemic lupus erythematosus27-30 and APS.4,5
β2-GPI is a molecule that is recruited to the surface of human apoptotic or modified cells. Here we define the function of β2-GPI as a human regulator of the complement activation pathway. On binding to apoptotic particles, β2-GPI changes the conformation from an inactive circular to an elongated form that acquires C3 binding activities. β2-GPI attached C3 provides binding sites for factor H that consequently mediate degradation of C3 by factor I. These new findings about β2-GPI are discussed in light of the autoimmune disease APS.
Methods
Antibodies and proteins
Plasma-derived purified β2-GPI was purchased from Scipac. Polyclonal antibodies against β2-GPI were raised by immunizing rabbits with purified β2-GPI (Eurogentec). Blood was obtained from healthy human donors on informed consent. The blood was centrifuged, and normal human serum (NHS) was stored at −80°C until use. For immune detection, polyclonal goat C3 antiserum, polyclonal rabbit C3a antiserum, monoclonal C3a antibodies, polyclonal goat factor B antiserum, and polyclonal rabbit anti–human SC5b-9neo (lgG, all CompTech) were used. β2-GPI antiserum (Eurogentec) and monoclonal β2-GPI antibody (1D2, Santa Cruz Biotechnology) were used for detection of β2-GPI, factor H/1–4 antiserum, or monoclonal C18 from Enzo Lifesciences for detection of factor H and human C5b-9 monoclonal antibody (Dako Denmark) for detection of the terminal complement complex. C3 was obtained from Calbiochem, and C3b, C3a, factor B, factor P, factor D, factor H, and factor I were obtained from CompTech.
Expression of recombinant proteins
β2-GPI fragments were generated by PCR amplification from a human liver cDNA library (Invitrogen) using the following primers: β2-GPI/IV-V (P1 forward, 5′-CGAATTCTTAGGGAAGTAAAATGCCCATT-3′ and P2 reverse) and β2-GPI /I-IV (P3 forward and P4 reverse, 5′-GTCTAGAGATGCTTTACAACTTGGCATG-3′) subsequently cloned into expression vector pPICZαB (Invitrogen). The β2-GPI fragments were expressed as secreted His-tagged proteins in Pichia pastoris strain X33. All recombinant proteins were purified by nickel chelate affinity chromatography and concentrated in PBS (Millipore). Conformational conversion of β2-GPI from the open to the circular form was performed by pH change as previously described.18 Factor H fragment SCR11 to 15 was expressed as previously described.30
Cofactor assay
The effect of β2-GPI, β2-GPI/I-IV, or β2-GPI/IV-V alone or in combination with factor H on cofactor activity was measured in cofactor assays as previously described.31 Briefly, C3b (10 μg/mL), factor H (5 μg/mL), and factor I (0.72 μg/mL) and β2-GPI (20-400 μg/mL, 0.4-7.2μM), β2-GPI/I-IV (7-69 μg/mL), or β2-GPI/IV-V (14-140 μg/mL; each 0.4-3.6μM) were incubated at 37°C for 30 minutes, separated by SDS-PAGE, and further analyzed by Western blotting using a C3 specific antiserum (1:2000). For cofactor experiments with C3, C3 (10 μg/mL) was preincubated with β2-GPI (20-80 μg/mL), β2-GPI/I-IV (14-56 μg/mL), or β2-GPI/IV-V (7-28 μg/mL, each 0.35-1.4μM) for 20 minutes. Factor H (10 μg/mL) and factor I (0.175 μg/mL) were added, and cleavage products were detected by SDS-PAGE followed by immunoblotting with anti-C3 antiserum (1:2000) or monoclonal C3a antibodies (1:1000). Cofactor activity of apoptotic particle bound factor H alone or in combination with purified β2-GPI was assayed by incubating particles (1 × 108 cells) with constant amounts of factor H (100 μg/mL, 0.7μM) and increasing concentrations of β2-GPI (33-100 μg/mL; 0.7-1.8μM) for 1 hour at 30°C. Coated particles were washed, incubated with C3 (10 μg/mL) and factor I (10 μg/mL) for 30 minutes at 37°C, and centrifuged, and the supernatants were immunoblotted using a C3 specific antiserum (1:3000).
C3 convertase activity
To study the effect of β2-GPI on the activity of the C3 convertase, plasma (5%) was preincubated with β2-GPI (50 and 100 μg/mL; 0.9 and 1.8μM), β2-GPI/I-IV (10, 50, and 100 μg/mL; 0.3, 1.5, and 3μM), or β2-GPI/IV-V (50μg/mL; 2.7μM) factor H (50 μg/mL; 0.3μM), or complement factor H and factor H-related protein 1 (50 μg/mL; 1.8μM); subsequently, complement was activated using zymosan (1 mg; Sigma-Aldrich) for 20 minutes at 37°C in HEPES-EDTA buffer (20mM HEPES, 7mM MgCl2 144mM NaCl, 10mM EDTA, pH 7.4). The supernatant was separated by SDS-PAGE, and C3a was detected by immunoblotting using polyclonal C3a antiserum (1:2000).
Complement activation assay
Regulatory activities of β2-GPI, β2-GPI/I-IV, or β2-GPI/IV-V on the alternative and classic pathways were analyzed using a modification of a previously described assay by Roos et al.32 Microtiter plates (F96 Maxisorb, Nunc-Immuno Module) were coated with lipopolysaccharide (10 μg/mL) for activation of the alternative pathway or IgM (10 μg/mL) for the classic pathway overnight at 4°C, washed 4 times with PBS-Tween, and blocked for 1 hour at 37°C with Dulbecco PBS containing 1% BSA. NHS was diluted in HEPES-EDTA buffer (above) or GVB2+ buffer (Complement Technology) and incubated for 1 hour at 37°C. NHS (20%, alternative pathway, 1% classic pathway) was preincubated for 15 minutes at 37°C with β2-GPI (5-20 μg/mL), β2-GPI/I-IV (3.5-14 μg/mL), or β2-GPI/IV-V (1.8-7 μg/mL), or factor H (10-50 μg/mL), staphylococcal-complement-inhibitor (SCIN; 2.5-10 μg/mL), or BSA (10-50 μg/mL, each 0.1-0.4μM) and added to the precoated wells. Complement activation was measured at 492 nm using a human C5b-9 monoclonal antibody. Absorbance of activated NHS alone was set to 100%.
Binding assays
C3 or C3b (each 5 μg/mL) was immobilized on a MaxiSorp microtiter plate, and equimolar amounts of circular β2-GPI (25 μg/mL), β2-GPI/I-IV (17.5 μg/mL), or β2-GPI/IV-V (8.5 μg/mL, each 0.5μM) and increasing amounts of factor H (2-8 μg/mL; 12.5-50nM) were added, and parallel binding of β2-GPI and factor H to C3 was measured using polyclonal rabbit β2-GPI or factor H antiserum (1:1000). In addition, equimolar amounts of circular or open β2-GPI (25 and 50 μg/mL), β2-GPI/I-IV (17.5 and 35 μg/mL), or β2-GPI/IV-V (8.5 and 17 μg/mL, each 0.5 and 1μM) together with factor H (5 μg/mL) were incubated with immobilized C3b (5 μg/mL), and binding to C3b was measured.
Immunoprecipitation
Monoclonal C3a or monoclonal β2-GPI antibodies were immobilized to mixed protein A and G Sepharose beads (1:1; GE Healthcare) by incubation overnight at 4°C. Antibody loaded beads were incubated in normal human plasma (NHP; 50%) for 2 hours at 4°C. Bound proteins were eluted in buffer containing 0.1M glycine/0.5M NaCl, pH 2.7, and eluates were separated by SDS-PAGE, transferred to a membrane, and C3 was detected using C3 antiserum (1:2000) and β2-GPI using β2-GPI antiserum (1:1000).
Apoptotic particles
Human umbilical vein endothelial cells (2 × 107) were kept serum free for 12 to 16 hours before induction of apoptosis with staurosporine (0.4 μg/mL). After 16 hours, apoptotic particles were isolated from the supernatant by centrifugation (10 000g) and resolved in Dulbecco PBS. Apoptotic particles (1 × 106) were incubated with β2-GPI (1-100 μg/mL), β2-GPI/I-IV or β2-GPI/IV-V (each 1.8μM), or NHS (5%), and binding was detected by flow cytometry using β2-GPI antiserum (1:2000). Similarly, binding of C3b from NHS (5%) or purified C3b (1 μg/mL) to apoptotic particles was determined in the presence or absence of purified β2-GPI (100 μg/mL) using C3 antiserum (1:2000) for detection.
Apoptotic particles (1 × 106) were mounted on chamber slides (Nunc) incubated with β2-GPI (20 μg/mL; 0.4μM) and annexin V (50 μg/mL), and proteins were detected by β2-GPI antiserum (1:200) and annexin V antibodies (1:200), as well as fluorescent-labeled secondary antibodies (Alexa-488, 1:400; and Alexa-647, 1:400). Particles were treated with Mount Fluor (Pro Taqs) and investigated with a laser scanning microscope 510 Meta (Carl Zeiss). To assay β2-GPI complement-regulatory functions on apoptotic particles, β2-GPI (5-40 μg/mL; 0.1-0.4μM), and factor H (60 μg/mL) were loaded onto particles (1 × 106) and incubated with C3 (10 μg/mL) and factor I (5 μg/mL) for 10 minutes. Subsequently, C3 cleavage was investigated by SDS-PAGE and immunoblotting using C3 antiserum (1:2000)
Statistical analysis
Statistics were analyzed with Student t test. P values < .05 were considered significant.
Results
β2-GPI is a complement regulator
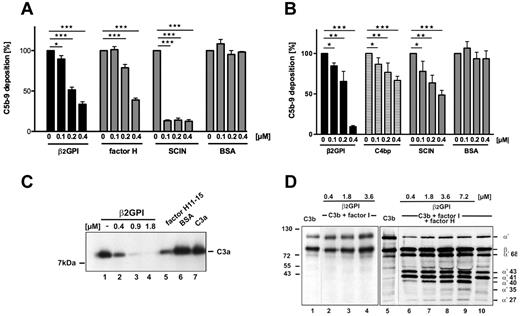
The structural similarity of β2-GPI and complement regulator factor H suggested that β2-GPI has complement-regulatory activities. To test this hypothesis, β2-GPI was added to NHS, complement was activated via the alternative pathway, and C5b-9 levels were determined by ELISA. β2-GPI inhibited complement activation, and C5b-9 generation was reduced by ∼ 63% (Figure 1A). The inhibitory activity was dose-dependent and similar to factor H and the bacterial complement inhibitor SCIN (Figure 1A). No inhibition by addition of BSA confirmed the specificity of the assay. β2-GPI also inhibited complement activation induced via the classic pathway in NHS (Figure 1B). Thus, β2-GPI is a regulator of both complement pathways.
β2-GPI regulates alternative and classic complement pathways. (A) NHS (20%) was preincubated with β2-GPI (0.1-0.4μM), and complement activation via the alternative pathway was assayed by determination of deposition of C5b-9. In the presence of β2-GPI, C5b-9 deposition was reduced (black columns). Factor H inhibited and bacterial inhibitor SCIN blocked complement activation (gray columns). BSA had no effect. (B) β2-GPI (black columns), C4BP (patterned columns), and bacterial inhibitor SCIN (gray columns) inhibited classic pathway activation in a dose-dependent manner. BSA had no effect. NHS (1%) was preincubated with equimolar amounts of β2-GPI, or C4BP, or SCIN or BSA (each 0.2-0.4μM) and added to IgM (10 μg/mL)–precoated wells. Complement activation was measured at 492 nm using a human C5b-9 monoclonal antibody. (A-B) Data are mean ± SD values of 3 independent experiments. *P < .05. **P < .01. ***P < .001. Activation of untreated NHS was set to 100%. (C) C3a is present in complement-activated NHS (lane 1). β2-GPI (0.4, 0.9, and 1.8μM; lanes 2-4) added to activated NHS inhibited complement activation on the level of C3a generation. Factor H/11–15 (1.8μM; lane 5) had minor effect and BSA (lane 1) had no effect. Purified C3a is shown in lane 7. (C) C3b (lane 1) is not cleaved by factor I in the presence of β2-GPI alone (lanes 2-4), but cleavage of C3b by factor I and cofactor factor H (lane 10) is enhanced in the presence of increasing amounts of β2-GPI (lanes 6-9), and additional cleavage products were generated. (C-D) Western blots show representative experiments of 3.
β2-GPI regulates alternative and classic complement pathways. (A) NHS (20%) was preincubated with β2-GPI (0.1-0.4μM), and complement activation via the alternative pathway was assayed by determination of deposition of C5b-9. In the presence of β2-GPI, C5b-9 deposition was reduced (black columns). Factor H inhibited and bacterial inhibitor SCIN blocked complement activation (gray columns). BSA had no effect. (B) β2-GPI (black columns), C4BP (patterned columns), and bacterial inhibitor SCIN (gray columns) inhibited classic pathway activation in a dose-dependent manner. BSA had no effect. NHS (1%) was preincubated with equimolar amounts of β2-GPI, or C4BP, or SCIN or BSA (each 0.2-0.4μM) and added to IgM (10 μg/mL)–precoated wells. Complement activation was measured at 492 nm using a human C5b-9 monoclonal antibody. (A-B) Data are mean ± SD values of 3 independent experiments. *P < .05. **P < .01. ***P < .001. Activation of untreated NHS was set to 100%. (C) C3a is present in complement-activated NHS (lane 1). β2-GPI (0.4, 0.9, and 1.8μM; lanes 2-4) added to activated NHS inhibited complement activation on the level of C3a generation. Factor H/11–15 (1.8μM; lane 5) had minor effect and BSA (lane 1) had no effect. Purified C3a is shown in lane 7. (C) C3b (lane 1) is not cleaved by factor I in the presence of β2-GPI alone (lanes 2-4), but cleavage of C3b by factor I and cofactor factor H (lane 10) is enhanced in the presence of increasing amounts of β2-GPI (lanes 6-9), and additional cleavage products were generated. (C-D) Western blots show representative experiments of 3.
β2-GPI regulates the C3 convertase of the alternative complement pathway
To further characterize the complement-regulatory functions of β2-GPI, we asked whether β2-GPI–like factor H inhibits the C3 convertase of the alternative pathway. Again, β2-GPI was added to NHS, complement was activated via the alternative pathway, and C3 convertase activity was assayed by the determination of C3a generation using Western blotting. β2-GPI inhibited C3a generation (Figure 1C). Specificity of β2-GPI function is concluded as addition of factor H/11–15 or BSA did not affect complement activation and C3a generation (Figure 1C). The results show that β2-GPI acts as complement regulator and suggest a regulation on level of the C3 convertase.
β2-GPI lacks decay accelerating activity of the assembled C3 convertase
To further define the mechanism how β2-GPI inhibits the C3 convertase activity, the effect of β2-GPI on the dissociation of the convertase complex, called decay accelerating activity, was tested. To this end, the C3 convertase C3bBb was formed on a surface by incubation of factor B, factor D, and properdin with immobilized C3b. β2-GPI did not dissociate the preformed C3 convertase. In the presence of β2-GPI, factor B/Bb remained attached to the C3 convertase, also when β2-GPI concentrations increased. In contrast, factor H showed decay-accelerating activity (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
β2-GPI enhances C3b cleavage by factor I
Showing lack of decay-accelerating activity, we next asked whether β2-GPI acts on the C3 convertase by mediating cofactor activity for the serine protease factor I to allow degradation of C3b. β2-GPI alone lacked cofactor activity for factor I and C3b remained intact (Figure 1D). However, when β2-GPI was added to C3b together with factor I and factor H, C3b cleavage was enhanced (Figure 1D). Additional C3b cleavage products with mobilities of 40, 35, and 27 kDa (Figure 1D) appeared. The sequence of these fragments was determined by mass spectrometry and revealed sequence identity to the C3 α' chain of C3b, C3c, and C3d (supplemental Table 1). β2-GPI processing by factor I was excluded (supplemental Figure 1B). These results suggest that β2-GPI modulates the C3b protein, which exposes new cleavage sites.
Elongated β2-GPI binds to C3b
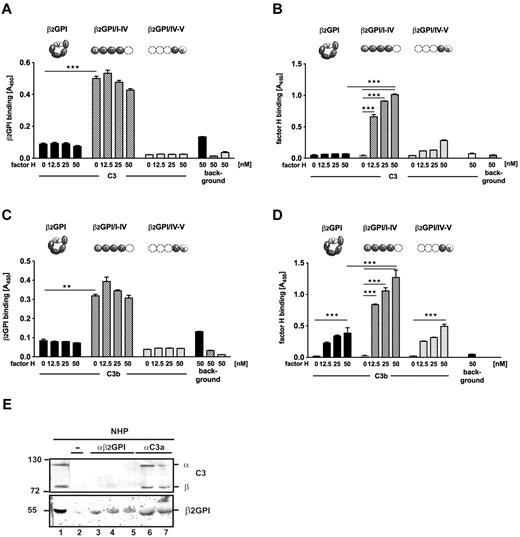
Because, in the presence of β2-GPI, new cleavage sites were exposed in the C3b molecule, we hypothesized that β2-GPI binds to C3b. Therefore, interaction of β2-GPI with C3b was assayed by ELISA. β2-GPI bound rather weakly to immobilized C3b (Figure 2A). However, β2-GPI exists in 2 conformations, a circular and elongated form, and we asked whether the 2 conformations differ in their C3b binding profiles. To this end, plasma purified β2-GPI, which represents predominantly the circular form, was treated transiently with a “high basic ” buffer (pH 11.5)18 to generate the elongated β2-GPI. So treated β2-GPI was assayed for C3b binding and showed low, but dose-dependent, binding to C3b (Figure 2A). To confirm binding of elongated β2-GPI to C3b and to localize the binding domain within the protein, 2 recombinant β2-GPI fragments composed of either the 4 N-terminal domains (β2-GPI/I-IV) or the 2 C-terminal domains (β2-GPI/IV-V) of β2-GPI were cloned, expressed in P pastoris, and purified (supplemental Figure 1C). Because of the absence of SCRV, fragment β2-GPI/I-IV did not form a circle. When assayed for C3b binding, the β2-GPI/I-IV fragment bound to C3b in a dose-dependent manner. In contrast, fragment β2-GPI/IV-V did not bind to C3b (Figure 2B). The N-terminal deletion mutant β2-GPI/I-IV bound stronger to immobilized C3b compared with the chemically modified β2-GPI. This difference in binding may be explained by the inefficient opening or refolding of the treated native protein. The results confirm binding of elongated β2-GPI to C3b and emphasize that the conformational change of β2-GPI from the circular to the elongated conformation is relevant for C3b binding. The results also demonstrate that elongated β2-GPI interacts via SCRI to SCRIII with C3b. To identify the C3 domain that interacts with β2-GPI, binding of the fragment β2-GPI/I-IV to C3 and the C3 cleavage products C3b, C3d, and iC3b was assayed by ELISA. β2-GPI/I-IV bound to C3, C3b, and iC3b, but not to C3d (supplemental Figure 2).
β2-GPI in its elongated form interacts with C3b and enhances degradation of C3b. (A) β2-GPI (black columns) shows minor binding to C3b, but pH-modified β2-GPI (gray columns) gained binding activities to C3b. Binding of the β2-GPI antibody to C3b is shown (background). (B) β2-GPI (black columns) shows minor binding to C3b, but recombinant β2-GPI/I-IV (patterned columns) bound to immobilized C3b. The binding was dose-dependent. β2-GPI/IV-V (light gray columns) did not bind to C3b. Data are mean ± SD of 3 independent experiments. *P < .05. ***P < .001. (C) NHS (20%) was preincubated with β2-GPI, β2-GPI/I-IV, or β2-GPI/IV-V (each 0.1-0.4μM), surface activated via the alternative complement pathway, and assayed for presence of C5b-9. β2-GPI (black columns) and β2-GPI/I-IV (patterned columns) reduced C5b-9 deposition. Fragment β2-GPI/IV-V (light gray columns) and BSA (gray columns) showed no effect on pathway activation. Data are mean plus or minus SD of 4 independent experiments. *P < .05. **P < .01. ***P < .001. (D) β2-GPI/I-IV showed low cofactor enhancing activities (lanes 2-5). (E) β2-GPI/IV-V did not modulate C3b cofactor activities (lanes 2-5).
β2-GPI in its elongated form interacts with C3b and enhances degradation of C3b. (A) β2-GPI (black columns) shows minor binding to C3b, but pH-modified β2-GPI (gray columns) gained binding activities to C3b. Binding of the β2-GPI antibody to C3b is shown (background). (B) β2-GPI (black columns) shows minor binding to C3b, but recombinant β2-GPI/I-IV (patterned columns) bound to immobilized C3b. The binding was dose-dependent. β2-GPI/IV-V (light gray columns) did not bind to C3b. Data are mean ± SD of 3 independent experiments. *P < .05. ***P < .001. (C) NHS (20%) was preincubated with β2-GPI, β2-GPI/I-IV, or β2-GPI/IV-V (each 0.1-0.4μM), surface activated via the alternative complement pathway, and assayed for presence of C5b-9. β2-GPI (black columns) and β2-GPI/I-IV (patterned columns) reduced C5b-9 deposition. Fragment β2-GPI/IV-V (light gray columns) and BSA (gray columns) showed no effect on pathway activation. Data are mean plus or minus SD of 4 independent experiments. *P < .05. **P < .01. ***P < .001. (D) β2-GPI/I-IV showed low cofactor enhancing activities (lanes 2-5). (E) β2-GPI/IV-V did not modulate C3b cofactor activities (lanes 2-5).
β2-GPI/I-IV harbors complement-regulatory activities
On the basis that fragment β2-GPI/I-IV bound to C3b, the complement-regulatory function of this recombinant deletion mutant was assayed. β2-GPI/I-IV was added to NHS, complement was activated via the alternative pathway, and activation was assayed by C5b-9 generation. Fragment β2-GPI/I-IV inhibited C5b-9 generation by ∼ 30%, whereas fragment β2-GPI/IV-V had no effect (Figure 2C). Inhibition was specific but not as pronounced as that mediated by full-length β2-GPI. Similar inhibitory effects were observed when complement was activated via the classic pathway. Thus, the N-terminal SCR domains I to III are central for the complement-regulatory activities; however, apparently also the C-terminal SCRV contributes to the complement inhibitory activity. The deletion fragment β2-GPI/I-IV also showed minor cofactor-enhancing activity, based on the increased amounts of cleavage products of 43 and 41 kDa (Figure 2D). Fragment β2-GPI/IV-V lacked this activity (Figure 2E). Thus, we conclude that β2-GPI regulates complement activation in its elongated conformation.
β2-GPI mediates cleavage of C3
The enforced C3b cleavage mediated by elongated β2-GPI suggested that elongated β2-GPI induces a conformational change of C3b by β2-GPI and raised the question whether β2-GPI also changes conformation of native C3. To test this hypothesis, β2-GPI was incubated with C3 and assayed for degradation. Indeed, in the presence of β2-GPI, C3 was cleaved by factor I and in the presence of cofactor factor H. Fragments with mobilities at 43 and 41 kDa were detected (Figure 3A). However, no cleavage product of 68 kDa, representing part of the α′ chain of C3b was detectable. To confirm similar cleavage of the C3 α chain, the same probes were developed with an antiserum specific for the C3a fragment. This antibody detected a 77-kDa fragment only when C3 was cleaved in the presence of β2-GPI (Figure 3B). This result confirmed cleavage of C3, as the C3a part of C3 remained attached to the cleavage fragment of 77 kDa. In the absence of β2-GPI, C3 was not degraded by factor I and factor H and very low background C3 cleavage occurred. In addition, C3 remained intact when incubated with a factor H fragment that encompasses SCR8 to SCR11 in the presence of factor H and factor I (supplemental Figure 3). Fragment β2-GPI/I-IV also mediated cleavage of C3 by factor I (Figure 3C) and cleavage products of 43 and 41 kDa were detected. Again, the C3a containing cleavage product appeared (Figure 3D). Fragment β2-GPI/IV-V induced no cleavage of C3 by factor I (Figure 3C). The results indicate that elongated, full-length β2-GPI induces an allosteric conformation of C3, which then exposes binding regions for factor H followed by the cleavage of C3 by factor I. This C3 transforming activity is mediated by the 3 N-terminal domains of β2-GPI but is most efficiently mediated by full length. However, no additional smaller cleavage products of C3 appeared, which could be explained by different effects of β2-GPI on C3 and C3b.
β2-GPI mediates inactivation of intact C3. (A) β2-GPI enabled degradation of C3 by factor I and factor H (lanes 4-6), but C3 was not cleaved by factor I in the presence of factor H (lane 2). The mobility of C3 cleavage products of 43 and 41 kDa is indicated. No cleavage product at 68 kDa was detected. (B) Probes from experiment shown in panel A were immunostained using a monoclonal C3a antibody. Cleavage product of 77 kDa, which represents the α-chain fragment including the C3a domain, is indicated. (C) Fragment β2-GPI/I-IV (lanes 8-10) mediated lower cleavage activities of C3 than β2-GPI and β2-GPI/IV-V (lanes 11-14) showed no effect. (D) C3a staining of the probes demonstrated a C3a containing cleavage product of 77 kDa in the presence of β2-GPI (lane 1) and β2-GPI/I-IV (lanes 3-5). Representative Western blots of 4 experiments are shown.
β2-GPI mediates inactivation of intact C3. (A) β2-GPI enabled degradation of C3 by factor I and factor H (lanes 4-6), but C3 was not cleaved by factor I in the presence of factor H (lane 2). The mobility of C3 cleavage products of 43 and 41 kDa is indicated. No cleavage product at 68 kDa was detected. (B) Probes from experiment shown in panel A were immunostained using a monoclonal C3a antibody. Cleavage product of 77 kDa, which represents the α-chain fragment including the C3a domain, is indicated. (C) Fragment β2-GPI/I-IV (lanes 8-10) mediated lower cleavage activities of C3 than β2-GPI and β2-GPI/IV-V (lanes 11-14) showed no effect. (D) C3a staining of the probes demonstrated a C3a containing cleavage product of 77 kDa in the presence of β2-GPI (lane 1) and β2-GPI/I-IV (lanes 3-5). Representative Western blots of 4 experiments are shown.
β2-GPI forms complexes with C3/C3b and factor H
To show recruitment of factor H to β2-GPI bound C3, complex formation between β2-GPI or β2-GPI fragments and factor H with C3 was analyzed. Therefore, C3 was immobilized to a surface and incubated with β2-GPI or β2-GPI fragments together with increasing amounts of factor H. Bound β2-GPI or factor H to C3 was determined by ELISA. Plasma-purified circular β2-GPI did not bind to C3. But fragment β2-GPI/I-IV bound to C3, and this binding was similar in the presence of increasing concentrations of factor H. C-terminal fragment β2-GPI/IV-V did not bind to C3 (Figure 4A). Measuring factor H binding in the complex revealed binding of factor H to C3 only when C3 was preincubated with β2-GPI/I-IV. Factor H bound in a dose-dependent manner. Thus, β2-GPI/I-IV formed a complex with C3 and factor H. This complex formation did not occur when C3 was incubated with circular β2-GPI or with β2-GPI/IV-V (Figure 4B). Factor H alone did not bind to C3 (Figure 4B) and also did not interact with β2-GPI or β2-GPI fragments (data not shown). In summary, β2-GPI/I-IV binds to C3, probably induces a conformational change of C3, which then exposes binding sites for factor H to form a complex.
β2-GPI forms complexes with C3/C3b and factor H. β2-GPI, β2-GPI/I-IV, and β2-GPI/IV-V together with increasing amounts of factor H were incubated with immobilized C3/C3b and binding of β2-GPI and in parallel of factor H were analyzed by ELISA. (A) β2-GPI/I-IV bound to immobilized C3, and binding was not increased with higher amounts of factor H present (patterned columns). β2-GPI and β2-GPI/IV-V showed no binding to C3 (black and light gray columns). Low binding of the ligands to the plate without C3 is shown (background). (B) β2-GPI/I-IV bound to C3 enabled dose-dependent binding of factor H to C3 (patterned columns). Circular β2-GPI had no effect on factor H binding to C3 (black columns) and fragment β2-GPI/IV-V mediated minor binding of factor H to C3 (light gray columns). Factor H binding to C3 alone (small black column) or to the plate (background) was excluded. (C) Steady amounts of β2-GPI/I-IV bound to C3b (patterned columns) in the presence of increasing concentrations of factor H. Circular β2-GPI (black columns) and β2-GPI/IV-V (light gray columns) did not bind to C3b. Binding of the proteins β2-GPI, β2-GPI/I-IV, and β2-GPI/IV-V without C3b (background). (D) In the presence of β2-GPI/I-IV, increasing amounts of factor H bound to C3b (patterned columns). Preincubation of C3b with circular β2-GPI (black columns) or β2-GPI/IV-V (light gray columns) did not enhance binding of factor H to C3b. (A-D) Data are mean ± SD of 3 independent experiments. **P < .01. ***P < .001. (E) β2-GPI/C3 complexes were isolated from NHP as β2-GPI (lanes 6 and 7 bottom panel) was bound to precipitated C3 (lanes 6 and 7 top panel) and showed similar mobility as plasma β2-GPI (lane 1 bottom panel). β2-GPI did not bind to the column alone (lane 2 bottom panel). NHP-derived C3 did not bind to immobilized β2-GPI (lanes 3-5 top panel).
β2-GPI forms complexes with C3/C3b and factor H. β2-GPI, β2-GPI/I-IV, and β2-GPI/IV-V together with increasing amounts of factor H were incubated with immobilized C3/C3b and binding of β2-GPI and in parallel of factor H were analyzed by ELISA. (A) β2-GPI/I-IV bound to immobilized C3, and binding was not increased with higher amounts of factor H present (patterned columns). β2-GPI and β2-GPI/IV-V showed no binding to C3 (black and light gray columns). Low binding of the ligands to the plate without C3 is shown (background). (B) β2-GPI/I-IV bound to C3 enabled dose-dependent binding of factor H to C3 (patterned columns). Circular β2-GPI had no effect on factor H binding to C3 (black columns) and fragment β2-GPI/IV-V mediated minor binding of factor H to C3 (light gray columns). Factor H binding to C3 alone (small black column) or to the plate (background) was excluded. (C) Steady amounts of β2-GPI/I-IV bound to C3b (patterned columns) in the presence of increasing concentrations of factor H. Circular β2-GPI (black columns) and β2-GPI/IV-V (light gray columns) did not bind to C3b. Binding of the proteins β2-GPI, β2-GPI/I-IV, and β2-GPI/IV-V without C3b (background). (D) In the presence of β2-GPI/I-IV, increasing amounts of factor H bound to C3b (patterned columns). Preincubation of C3b with circular β2-GPI (black columns) or β2-GPI/IV-V (light gray columns) did not enhance binding of factor H to C3b. (A-D) Data are mean ± SD of 3 independent experiments. **P < .01. ***P < .001. (E) β2-GPI/C3 complexes were isolated from NHP as β2-GPI (lanes 6 and 7 bottom panel) was bound to precipitated C3 (lanes 6 and 7 top panel) and showed similar mobility as plasma β2-GPI (lane 1 bottom panel). β2-GPI did not bind to the column alone (lane 2 bottom panel). NHP-derived C3 did not bind to immobilized β2-GPI (lanes 3-5 top panel).
To further characterize β2-GPI functions, similar binding measurements regarding complex formation were performed using the C3b molecule. C3b was immobilized to the surface and incubated with β2-GPI or β2-GPI fragments together with increasing amounts of factor H. Again, β2-GPI and factor H binding was determined by ELISA. Circular β2-GPI showed low binding to C3b in the presence of factor H but more of fragment β2-GPI/I-IV attached to C3b. This binding was not enhanced by factor H. β2-GPI/IV-V did not bind to C3b (Figure 4C). Determination of factor H binding in this assay revealed normal factor H binding to immobilized C3b. However, factor H binding was strongly enhanced when fragment β2-GPI/I-IV was also bound to C3b (Figure 4D).
β2-GPI forms complexes with C3 in plasma
To verify the existence of β2-GPI /C3 complexes in plasma, immunoprecipitation was performed. Sepharose beads coated with C3a or β2-GPI antibodies were incubated with NHP; and after extensive washing, elute fractions were investigated for the presence of C3 and β2-GPI by immuno-blotting. β2-GPI was precipitated with C3 from human plasma (Figure 4E bottom panel), and binding of plasma-derived C3 to the immobilized C3a antibodies was confirmed (Figure 4E top panel). Thus, β2-GPI /C3 complexes were formed with plasma proteins. However, β2-GPI antibody immobilized plasma β2-GPI did not precipitate C3 from the plasma.
β2-GPI binds to apoptotic particles and recruits C3/C3b for inactivation
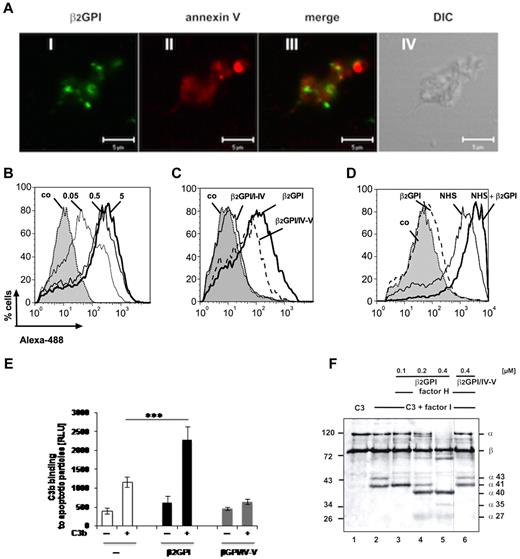
β2-GPI preferentially binds via the C-terminus to oxidized phospholipids on apoptotic cells and thereby opens the circular structure, leaving the N-terminus in solution to interact.16,18 Thus, we aimed to show the here identified complement-regulatory functions by β2-GPI when β2-GPI was attached to apoptotic surfaces. First, binding of β2-GPI to apoptotic surfaces was investigated. Apoptotic particles from human umbilical vein endothelial cells were generated as demonstrated by the binding of the natural ligand annexin V to phosphatidylserine on their outer surfaces using laser scanning microscopy (Figure 5A red fluorescence). Specific binding of β2-GPI to the apoptotic particles was visualized by laser scanning microscopy (Figure 5A green fluorescence). β2-GPI binding to apoptotic particles was also quantified by flow cytometry and revealed dose-dependent binding of β2-GPI to the particles (Figure 5B). To determine the binding site in β2-GPI for this attachment binding of β2-GPI, β2-GPI/I-IV, and β2-GPI/IV-V to the particles was assayed. Fragment β2-GPI/IV-V bound to the particles (Figure 5C), confirming previous results showing that the C-teminal part of the protein is responsible for surface attachment.16,18 Fragment β2-GPI/I-IV lacked binding activity. To investigate whether plasma C3/C3b spontaneously binds to the particles for opsonization, the particles were incubated in NHS, and deposition of C3/C3b was followed by flow cytometry. C3/C3b from serum bound to the particles (Figure 5D). However, addition of purified β2-GPI to the serum enhanced binding of C3/C3b to the particles (Figure 5D). To confirm recruitment of C3/C3b by particle-attached β2-GPI, β2-GPI and β2-GPI/IV-V were bound to particles and binding of purified C3b was assayed by flow cytometry. β2-GPI on the surface of the apoptotic particles attracted significantly more C3b to the surface than compared with fragment β2-GPI/IV-V. (Figure 5E) These results demonstrate that β2-GPI binds via the C-terminus to apoptotic particles and recruits C3/C3b from the plasma to the surface.
β2-GPI binds to apoptotic particles, recruits C3/C3b, and mediates inactivation by factor H and factor I. (A) β2-GPI (i, green fluorescence) and also annexin V (ii, red fluorescence) bound to apoptotic particles from human umbilical vein endothelial cells. Overlay demonstrates colocalization of β2-GPI and annexin V to particles (iii), and unstained particles are shown by differential interference contrast (DIC; iv) by laser scanning microscopy (LSM 510 META, Zeiss, oil immersion objective 63×/1.4 NA, LSM519 Version 3.2 software). Bars represent 5μm. (B) β2-GPI (0.05-5 μg) bound to the particles in a dose-dependent manner as measured by flow cytometry. (C) β2-GPI and β2-GPI/IV-V bound to apoptotic particles, in contrast to fragment β2-GPI/I-IV, which has the C-terminal SCR5 deleted. (D) C3/C3b from NHP is deposited on apoptotic particles and addition of β2-GPI to the plasma enhanced C3/C3b binding to the particles. (E) Purified C3b is recruited by β2-GPI (black columns) but not by β2-GPI/IV-V (gray columns) to the surface of apoptotic particles. Data are mean ± SD of 3 independent experiments. ***P < .001. (F) Apoptotic particles were loaded with increasing amounts of β2-GPI and constant amounts of factor H. C3 and factor I were added and C3 cleavage followed by immunoblotting. β2-GPI enhanced degradation of C3 (lanes 3-5). Novel cleavage products are indicated. Factor I alone (lane 2) or β2-GPI/IV-V (lane 6) had no enhancing effect. (A-C,E) Data are representative results from 3 experiments.
β2-GPI binds to apoptotic particles, recruits C3/C3b, and mediates inactivation by factor H and factor I. (A) β2-GPI (i, green fluorescence) and also annexin V (ii, red fluorescence) bound to apoptotic particles from human umbilical vein endothelial cells. Overlay demonstrates colocalization of β2-GPI and annexin V to particles (iii), and unstained particles are shown by differential interference contrast (DIC; iv) by laser scanning microscopy (LSM 510 META, Zeiss, oil immersion objective 63×/1.4 NA, LSM519 Version 3.2 software). Bars represent 5μm. (B) β2-GPI (0.05-5 μg) bound to the particles in a dose-dependent manner as measured by flow cytometry. (C) β2-GPI and β2-GPI/IV-V bound to apoptotic particles, in contrast to fragment β2-GPI/I-IV, which has the C-terminal SCR5 deleted. (D) C3/C3b from NHP is deposited on apoptotic particles and addition of β2-GPI to the plasma enhanced C3/C3b binding to the particles. (E) Purified C3b is recruited by β2-GPI (black columns) but not by β2-GPI/IV-V (gray columns) to the surface of apoptotic particles. Data are mean ± SD of 3 independent experiments. ***P < .001. (F) Apoptotic particles were loaded with increasing amounts of β2-GPI and constant amounts of factor H. C3 and factor I were added and C3 cleavage followed by immunoblotting. β2-GPI enhanced degradation of C3 (lanes 3-5). Novel cleavage products are indicated. Factor I alone (lane 2) or β2-GPI/IV-V (lane 6) had no enhancing effect. (A-C,E) Data are representative results from 3 experiments.
β2-GPI binds to apoptotic particles and mediates degradation of C3
To further investigate complement-regulatory activities of β2-GPI, which is bound to the apoptotic particles, β2-GPI and factor H were attached to the particles, washed in buffer, and C3 cleavage by factor I was followed by Western blotting. Bound β2-GPI mediated enhanced degradation of C3, as seen by the disappearance of the C3 α chain and the appearance of novel C3 cleavage products of ∼ 40, 36, and 27 kDa (Figure 5F). In contrast to β2-GPI, binding of the C-terminal fragment β2-GPI/IV-V together with factor H to the particles did not mediate cleavage of C3 to smaller fragments (Figure 5F). Thus, β2-GPI exerts complement-regulatory activities when bound to apoptotic particles in the form of recruiting C3 to the particles and mediation of degradation of C3 to C3d with the help of factor H and factor I.
Discussion
β2-GPI, the major target of autoantibodies in autoimmune disease antiphopspolipid syndrome, harbors complement-regulatory activities. Here we characterize β2-GPI as a complement regulator that inactivates C3 by a novel mechanism. Elongated but not circular β2-GPI, which is generated on the surface of apoptotic particles, recruits C3 and apparently induces a conformational change in the C3 molecule. Subsequently, C3 exposes binding sites for factor H and is cleaved by factor I. Thus, β2-GPI bound to apoptotic surfaces favors opsonization with C3 but inhibits further C3 convertase generation and complement activation.
β2-GPI is a complement regulator that inhibits complement activation by 2 different reactions. β2-GPI mediates C3 degradation and enhances C3b cleavage. β2-GPI binds to C3 and mediates inactivation by factor I and factor H. β2-GPI by itself lacks cofactor activity for factor I but induces a conformational change in C3, which enables binding of factor H to C3. Processing of the C3 molecule by complement regulators has not been described so far; thus, β2-GPI represents a novel type of human complement regulator. Based on the observation that β2-GPI acts like a special cofactor for factor H and factor I, we termed this function “cohancing” activity. β2-GPI binds C3/C3b outside the thioester-containing-domain (TED), as β2-GPI did not bind to C3d. How β2-GPI binds C3 in detail and induces allosteric changes is the subject of further investigations. Cohancing activity is dependent on the conformation of β2-GPI, as only the elongated form of β2-GPI mediates this activity. β2-GPI has 2 conformations: a circular and an elongated form.16,18 The circular form of β2-GPI represents the inactive form and is the predominant form of β2-GPI in plasma. On binding to specific surfaces, such as apoptotic cells, β2-GPI binds and changes its conformation to a J-shaped molecule, which binds C3/C3b (this study) as well as autoantibodies.18 The need of this conformational change of β2-GPI for C3 binding is confirmed by binding of recombinant fragment β2-GPI/I-IV, which is unable to form circles, to C3 and C3b. Circular β2-GPI showed very low binding to C3 but displayed cohancing activity, thus indicating that a fraction of circular β2-GPI was in the elongated conformation. In plasma, ∼ 0.1% of β2-GPI is in the elongated conformation.18 This β2-GPI immunoprecipitated with C3 from human plasma, when C3 was catched by antiC3a, which confirmed complex formation of plasma β2-GPI with C3. As expected, immobilized circular plasma β2-GPI by antibodies did not precipitate C3. The C-terminal end of β2-GPI alone had a minor effect on C3, which is in agreement with the cell surface binding function that is located, as factor H in this domain.19,20,33 However, as the elongated plasma purified β2-GPI revealed higher complement-regulatory activities compared with the recombinant fragment β2-GPI/I-IV, the C-terminus of β2-GPI supports the regulatory function of the protein. Altogether, the data show that β2-GPI in its elongated conformation acts as complement regulator.
β2-GPI enhances degradation of C3b. Besides the known C3b cleavage products of 68, 43, and 41 kDa by factor I/factor H, the presence of β2-GPI resulted in novel C3b cleavage products of ∼ 40, 35, and 27 kDa, which resemble the cleavage pattern generated by factor I in the presence of complement receptor 1.34 Complement receptor 1 mediates cleavage of C3b at position R932 in addition to factor H-mediated cleavage of C3b at positions R1281 and R1298. This indicates that β2-GPI also effects the conformation of the C3b molecule, which subsequently provides an additional cleavage site for factor I. Again, only β2-GPI in its elongated conformation showed C3b binding activities. β2-GPI inhibits generation of C3a in complement-activated NHP, which confirms the findings that β2-GPI inhibits complement amplification via factor H/factor I-mediated cohancing activity on C3 and C3b.
The identification of β2-GPI as complement regulator has implications for the autoimmune disease APS and recurrent fetal loss. Autoantibodies directed against β2-GPI bind to the open form of β2-GPI within the N-terminal SCR1,35,36 which harbors the here identified complement-regulatory functions. Indeed, several indications point to a complement deregulation in APS, such as vascular thrombosis, which is a common feature of other complement-associated diseases,23 or such as hypocomplementemia, reflecting enhanced complement activation and consumption.37,38 Inhibition of complement at the level of C3, C5, or C5a in mice prevents thrombosis, which clearly demonstrates a contribution of complement activation to the pathogenesis of β2-GPI–autoantibody-mediated thrombophilia.38,39 In addition, Holers et al40 identified a massive accumulation of complement component C3 in the placenta of mice with APS and showed that inhibition of C3 activation prevented fetal loss. In addition, mice that are blocked in alternative pathway activation either by factor B deficiency or by specific antibodies were protected from β2-GPI autoantibody-mediated fetal injury.41 Very recently, Lonze et al42 reported the successful renal transplantation in a patient with catastrophic APS when complement was inhibited via a C5 monoclonal antibody (eculizumab). Altogether, these observations support the hypothesis that inhibition of β2-GPI–mediated complement-regulatory functions by β2-GPI autoantibodies may represent the underlying mechanism for the accumulation of C3 activation products in the placenta, leading to the recruitment and activation of neutrophils.43 The understanding of β2-GPI as a complement-regulatory glycoprotein further emphasizes the central role of complement regulation in APS pathology. In addition, this role of β2-GPI is in agreement with the observation that thrombus formation induced by β2-GPI autoantibodies is complement dependent and requires a priming factor that activates complement.44
In conclusion, plasma glycoprotein β2-GPI represents a complement regulator of a novel type. When opened from the inactive circular to the elongated form, β2-GPI inactivates complement activation at the level of C3, before the convertases are formed, and enhances cleavage of C3/C3b by factor I (Figure 6). Interestingly, a related type of allosteric inhibition of C3b was also observed for the microbial complement inhibitor Efb secreted by Staphylococcus aureus.45 This analogy suggests that pathogenic microbes, such as S aureus, mimic human complement-regulatory proteins, such as β2-GPI, for immune protection.
β2-GPI mediates complement-regulatory activities. Circular, inactive β2-GPI is recruited from plasma to modified surfaces that expose oxidized phospholipids, such as cardiolipin. Circular β2-GPI opens up and can bind C3, inducing a conformational change to enable binding of factor H and degradation by factor I. Elongated β2-GPI also acts on C3b to enhance binding of factor H and degradation and inactivation by factor I.
β2-GPI mediates complement-regulatory activities. Circular, inactive β2-GPI is recruited from plasma to modified surfaces that expose oxidized phospholipids, such as cardiolipin. Circular β2-GPI opens up and can bind C3, inducing a conformational change to enable binding of factor H and degradation by factor I. Elongated β2-GPI also acts on C3b to enhance binding of factor H and degradation and inactivation by factor I.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sascha Böhm (Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany) for providing the recombinant SCIN protein and Andrea Hartmann for excellent technical assistance.
This work is supported by the Deutsche Forschungsgemeinschaft (Sk46) and the ProRetina Foundation. I.K. is a PhD student of the International Leibniz Research School for Microbial and Biomolecular Interactions Jena (Jena School for Microbial Communication, Priority Program 1160 of the Deutsche Forschungsgemeinschaft).
Authorship
Contribution: K.G., N.W., M.R., S.M., I.K., and T.H. performed the experiments and discussed the data; and C.S. conceived the experiments, discussed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christine Skerka, Department of Infection Biology Leibniz Institute for Natural Product Research and Infection Biology, Hans Knöll Institute, Beutenbergstrasse 11, D-07745 Jena, Germany; e-mail: christine.skerka@hki-jena.de.