Abstract
We evaluated spleen function in 193 children with sickle cell anemia 8 to 18 months of age by 99mTc sulfur-colloid liver-spleen scan and correlated results with clinical and laboratory parameters, including 2 splenic biomarkers: pitted cell counts (PIT) and quantitative Howell-Jolly bodies (HJB) enumerated by flow cytometry. Loss of splenic function began before 12 months of age in 86% of infants in association with lower total or fetal hemoglobin and higher white blood cell or reticulocyte counts, reinforcing the need for early diagnosis and diligent preventive care. PIT and HJB correlated well with each other and liver-spleen scan results. Previously described biomarker threshold values did define patients with abnormal splenic function, but our data suggest that normal spleen function is better predicted by PIT of ≤ 1.2% or HJB ≤ 55/106 red blood cells and absent function by PIT ≥ 4.5% or HJB ≥ 665/106. HJB is methodologically advantageous compared with PIT, but both are valid biomarkers of splenic function. This trial was registered at www.clinicaltrials.gov as #NCT00006400.
Introduction
The spleen is one of the first organs damaged in sickle cell anemia (SCA). Consequences of this damage, increased risk of invasive pneumococcal infection and splenic sequestration, are difficult to predict for a given child. The “gold standard” for assessment of splenic filtrative function is a 99mTc sulfur-colloid liver-spleen (LS) scan. However, LS scans yield qualitative results and require low-dose radiation exposure.
BABY HUG, the Pediatric Hydroxyurea phase 3 Clinical Trial, is an National Heart, Lung, and Blood Institute and National Institute of Child Health and Human Development–sponsored 14-center, randomized double-blind placebo-controlled trial of the efficacy of hydroxyurea in preventing chronic organ damage in infants with SCA. The trial was approved by the institutional review boards of all participating centers. Prevention of loss of splenic function is a primary endpoint.1
After written informed consent in accordance with the Declaration of Helsinki, splenic filtrative function of children in BABY HUG was assessed by LS scan and compared with 2 surrogate biomarkers: pitted erythrocyte (PIT) counts by Nomarski optics and Howell-Jolly Bodies (HJBs) quantitated by flow cytometry. These pretreatment baseline data form the largest reported assessment of spleen function in young children with SCA by LS scan, define parameters of loss of function, and validate both biomarkers as accurate noninvasive measures of splenic function.
Methods
LS scan
A standardized dose of 0.05 mCi/kg (minimum 0.5 mCi, 1 mCi if local practice) of 99mTc sulfur-colloid was injected into a peripheral vein. Liver and spleen were imaged 30 minutes later in the posterior, anterior, left anterior oblique, and right posterior oblique planes. Splenic uptake was qualitatively interpreted on de-identified films by structured consensus of 3 pediatric nuclear medicine physicians as normal (spleen uptake equal to liver on posterior image and/or left lobe on left anterior oblique), present but decreased (less uptake in the spleen compared with liver), or absent (no uptake in the spleen).
PIT counts
Red cell membrane vacuoles occur as cells age, and resultant “pitted” red cells are culled by splenic macrophages. Specimens prepared locally by fixing one or 2 drops of blood in 0.5 mL of 1% buffered glutaraldehyde were shipped to a central laboratory. The percentage of 500 erythrocytes with at least one vacuole was reported as the PIT count.2-5 A PIT count more than 3.5% has been considered to be indicative of loss of splenic filtrative function in SCA.4
Quantitative HJB
HJB were quantitated in a central laboratory by flow cytometric evaluation of at least 106 mature (CD71−) erythrocytes (Litron Laboratories).6 Higher proportions of HJB in peripheral blood suggest a decline in spleen function. Values > 300/106 erythrocytes have been associated with loss of splenic filtrative function.7,8
Associations
LS scan uptake was correlated with age and compared with PIT and HJB. Relationships were explored between total hemoglobin (Hb) and fetal (HbF) hemoglobin, white blood cells (WBC), absolute neutrophil (ANC), and absolute reticulocyte (ARC) counts; prior clinical events, including splenic sequestration, dactylitis, vaso-occlusive crises, palpable spleen, transfusion, hospitalization other than for fever; and highest time-averaged mean-maximum transcranial Doppler velocity,9 glomerular filtration rate determined by 99mTc-DTPA clearance (DTPA-GFR),10 transcutaneous oxygen saturation (O2), and spleen volume (SVOL by US) calculated from ultrasound dimensions using the formula for an ellipsoid: [(length × anterior-posterior × transverse) × 0.523].11
Logarithmic transformation was applied to each parameter (except age) to improve linearity with other variables and stabilize the variance of the transformed data.
Results and discussion
An LS scan was performed on 193 infants with SCA (58% female; mean age, 12.9 months; range, 8.1-18 months) not selected for clinical severity and more than 2 months from last transfusion. Twenty-three children (12%) had normal splenic uptake, 142 (73%) present but decreased uptake, and 28 (15%) absent uptake. Absent splenic function was associated with a higher mean age (P = .001). No age-defined subgroup had more than 25% of patients with normal splenic function (Table 1).
Spleen uptake on LS scan
| . | Normal . | Decreased . | Absent . | P* . | CCR, % . |
|---|---|---|---|---|---|
| BABY HUG splenic function data | |||||
| No. (%) (N = 193) | 23 (12%) | 142 (73%) | 28 (15%) | — | — |
| Age, mo (range, 8.1-18) (mean ± SD) | 12.2 ± 2.5 | 12.7 ± 2.5 | 14.6 ± 2.9 | .001 | — |
| ≤ 9.0 mo (N = 12) | 3 (25%) | 8 (67%) | 1 (8%) | — | — |
| 9.1-12 mo (N = 69) | 10 (14%) | 53 (77%) | 6 (9%) | — | — |
| 12.1-15 mo (N = 68) | 6 (9%) | 55 (81%) | 7 (10%) | — | — |
| ≥ 15.1 mo (N = 44) | 4 (9%) | 26 (59%) | 14 (32%) | — | — |
| Blood counts (mean ± SD) | |||||
| Hb, g/dL | 9.9 ± 1.2 | 9.1 ± 1.2 | 8.2 ± 1.0 | < .001 | — |
| Hb F, % | 34.1 ± 6.7 | 26.2 ± 8.1 | 19.2 ± 6.5 | < .001 | — |
| ARC, × 109/L | 194 ± 62 | 301 ± 140 | 366 ± 110 | < .001 | — |
| WBC, × 109/L | 10.9 ± 3.3 | 13.9 ± 5.5 | 18.7 ± 5.6 | < .001 | — |
| ANC, × 109/L | 3.0 ± 1.1 | 4.2 ± 2.3 | 5.6 ± 2.7 | .007 | — |
| Clinical events, % of patients | — | ||||
| Vaso-occlusive events | 21.7 | 48.2 | 75.0 | < .001* | — |
| Dactylitis | 21.7 | 35.3 | 64.3 | .004* | — |
| Hospitalization not for fever | 0 | 26.4 | 40.0 | .009* | — |
| Transfusion | 0 | 13.2 | 29.6 | .006* | — |
| Acute splenic sequestration | 0 | 6.6 | 22.2 | .012* | — |
| Palpable spleen | 0 | 19.7 | 28.6 | .012* | — |
| Organ function | |||||
| TCD velocity, maximal mean (cm/s) | 106 ± 23 | 122 ± 20 | 136 ± 20 | < .001 | — |
| DTPA-GFR, mL/min per 1.73 m2 | 129 ± 48 | 124 ± 31 | 119 ± 31 | .650 | — |
| SVOL by US, mL | 94 ± 38 | 107 ± 44 | 95 ± 51 | .103 | — |
| O2 saturation, % | 98.5 | 98.9 | 98.9 | .469 | — |
| Biomarkers | |||||
| PIT, % | 1.1 ± 0.9 | 4.1 ± 4.6 | 10.1 ± 5.3 | < .001 | — |
| < 3.5% (N = 112) | 23 | 85 | 4 | — | — |
| ≥ 3.5% (N = 74) | 0 | 52 | 22 | — | — |
| ≤ 1.0% (N = 49) | 14 | 34 | 1 | — | — |
| > 1.0%-< 5.2% (N = 80) | 9 | 67 | 4 | — | — |
| ≥ 5.2% (N = 57) | 0 | 36 | 21 | — | — |
| PIT: CCR normal versus all other | — | — | — | — | 65 |
| ≤ 1.2% (N = 54) | 15 | 38 | 1 | — | — |
| > 1.2% (N = 132) | 8 | 99 | 25 | — | — |
| PIT: CCR absent versus all other | — | — | — | — | 81 |
| < 4.5% (N = 122) | 23 | 94 | 5 | — | — |
| ≥ 4.5% (N = 64) | 0 | 43 | 21 | — | — |
| HJB (per 106 RBC) | 49 ± 59 | 501 ± 683 | 1491 ± 707 | < .001 | — |
| < 300 (N = 105) | 23 | 82 | 0 | — | — |
| ≥ 300 (N = 77) | 0 | 53 | 24 | — | — |
| ≤ 42 (N = 68) | 18 | 50 | 0 | — | — |
| > 42-< 678 (N = 50) | 5 | 44 | 1 | — | — |
| ≥ 678 (N = 64) | 0 | 41 | 23 | — | — |
| HJB: CCR normal versus all other | — | — | — | 83 | |
| ≤ 55 (N = 80) | 19 | 61 | 0 | — | — |
| > 55 (N = 102) | 4 | 74 | 24 | — | — |
| HJB: CCR absent versus all other | — | — | — | — | 96 |
| < 665 (N = 118) | 23 | 94 | 1 | — | — |
| ≥ 665 (N = 64) | 0 | 41 | 23 | — | — |
| Cooperative Study of Sickle Cell Disease data4 | |||||
| No. of patients (% of total) | 44 (69%) | 5 (8%) | 15 (23%) | — | — |
| Age, mo (range) | 9.7 (8-13) | 10.9 (8-13) | 13.3 (11-13) | — | — |
| Hb, g/dL | 9.9 ± 1.0 | 9.6 ± 2.1 | 8.3 ± 1.3 | — | — |
| Hb F, % | 23.6 ± 12.4 | 26.0 ± 11.6 | 13.7 ± 6.5 | — | — |
| PIT, % | 1.9 ± 1.0 | 3.7 ± 4.3 | 7.9 ± 3.2 | — | — |
| < 3.5% (N = 45) | 43 | 2 | 0 | — | — |
| ≥ 3.5% (N = 19) | 1 | 3 | 15 | — | — |
| . | Normal . | Decreased . | Absent . | P* . | CCR, % . |
|---|---|---|---|---|---|
| BABY HUG splenic function data | |||||
| No. (%) (N = 193) | 23 (12%) | 142 (73%) | 28 (15%) | — | — |
| Age, mo (range, 8.1-18) (mean ± SD) | 12.2 ± 2.5 | 12.7 ± 2.5 | 14.6 ± 2.9 | .001 | — |
| ≤ 9.0 mo (N = 12) | 3 (25%) | 8 (67%) | 1 (8%) | — | — |
| 9.1-12 mo (N = 69) | 10 (14%) | 53 (77%) | 6 (9%) | — | — |
| 12.1-15 mo (N = 68) | 6 (9%) | 55 (81%) | 7 (10%) | — | — |
| ≥ 15.1 mo (N = 44) | 4 (9%) | 26 (59%) | 14 (32%) | — | — |
| Blood counts (mean ± SD) | |||||
| Hb, g/dL | 9.9 ± 1.2 | 9.1 ± 1.2 | 8.2 ± 1.0 | < .001 | — |
| Hb F, % | 34.1 ± 6.7 | 26.2 ± 8.1 | 19.2 ± 6.5 | < .001 | — |
| ARC, × 109/L | 194 ± 62 | 301 ± 140 | 366 ± 110 | < .001 | — |
| WBC, × 109/L | 10.9 ± 3.3 | 13.9 ± 5.5 | 18.7 ± 5.6 | < .001 | — |
| ANC, × 109/L | 3.0 ± 1.1 | 4.2 ± 2.3 | 5.6 ± 2.7 | .007 | — |
| Clinical events, % of patients | — | ||||
| Vaso-occlusive events | 21.7 | 48.2 | 75.0 | < .001* | — |
| Dactylitis | 21.7 | 35.3 | 64.3 | .004* | — |
| Hospitalization not for fever | 0 | 26.4 | 40.0 | .009* | — |
| Transfusion | 0 | 13.2 | 29.6 | .006* | — |
| Acute splenic sequestration | 0 | 6.6 | 22.2 | .012* | — |
| Palpable spleen | 0 | 19.7 | 28.6 | .012* | — |
| Organ function | |||||
| TCD velocity, maximal mean (cm/s) | 106 ± 23 | 122 ± 20 | 136 ± 20 | < .001 | — |
| DTPA-GFR, mL/min per 1.73 m2 | 129 ± 48 | 124 ± 31 | 119 ± 31 | .650 | — |
| SVOL by US, mL | 94 ± 38 | 107 ± 44 | 95 ± 51 | .103 | — |
| O2 saturation, % | 98.5 | 98.9 | 98.9 | .469 | — |
| Biomarkers | |||||
| PIT, % | 1.1 ± 0.9 | 4.1 ± 4.6 | 10.1 ± 5.3 | < .001 | — |
| < 3.5% (N = 112) | 23 | 85 | 4 | — | — |
| ≥ 3.5% (N = 74) | 0 | 52 | 22 | — | — |
| ≤ 1.0% (N = 49) | 14 | 34 | 1 | — | — |
| > 1.0%-< 5.2% (N = 80) | 9 | 67 | 4 | — | — |
| ≥ 5.2% (N = 57) | 0 | 36 | 21 | — | — |
| PIT: CCR normal versus all other | — | — | — | — | 65 |
| ≤ 1.2% (N = 54) | 15 | 38 | 1 | — | — |
| > 1.2% (N = 132) | 8 | 99 | 25 | — | — |
| PIT: CCR absent versus all other | — | — | — | — | 81 |
| < 4.5% (N = 122) | 23 | 94 | 5 | — | — |
| ≥ 4.5% (N = 64) | 0 | 43 | 21 | — | — |
| HJB (per 106 RBC) | 49 ± 59 | 501 ± 683 | 1491 ± 707 | < .001 | — |
| < 300 (N = 105) | 23 | 82 | 0 | — | — |
| ≥ 300 (N = 77) | 0 | 53 | 24 | — | — |
| ≤ 42 (N = 68) | 18 | 50 | 0 | — | — |
| > 42-< 678 (N = 50) | 5 | 44 | 1 | — | — |
| ≥ 678 (N = 64) | 0 | 41 | 23 | — | — |
| HJB: CCR normal versus all other | — | — | — | 83 | |
| ≤ 55 (N = 80) | 19 | 61 | 0 | — | — |
| > 55 (N = 102) | 4 | 74 | 24 | — | — |
| HJB: CCR absent versus all other | — | — | — | — | 96 |
| < 665 (N = 118) | 23 | 94 | 1 | — | — |
| ≥ 665 (N = 64) | 0 | 41 | 23 | — | — |
| Cooperative Study of Sickle Cell Disease data4 | |||||
| No. of patients (% of total) | 44 (69%) | 5 (8%) | 15 (23%) | — | — |
| Age, mo (range) | 9.7 (8-13) | 10.9 (8-13) | 13.3 (11-13) | — | — |
| Hb, g/dL | 9.9 ± 1.0 | 9.6 ± 2.1 | 8.3 ± 1.3 | — | — |
| Hb F, % | 23.6 ± 12.4 | 26.0 ± 11.6 | 13.7 ± 6.5 | — | — |
| PIT, % | 1.9 ± 1.0 | 3.7 ± 4.3 | 7.9 ± 3.2 | — | — |
| < 3.5% (N = 45) | 43 | 2 | 0 | — | — |
| ≥ 3.5% (N = 19) | 1 | 3 | 15 | — | — |
— indicates not applicable; CCR, correct classification rate; US, ultrasound; and TCD, transcranial Doppler.
P values are derived from χ2 test or Fisher exact test. All other P values are derived from F test statistic calculated on the transformed variable comparing distributions among the 3 groups.
HJB (P < .001) and PIT (P < .001) were positively associated with increasing age. Hb (P = .015) and HbF (P < .001) values decreased, whereas WBC (P = .014) and ARC (P = .036) increased with older age.
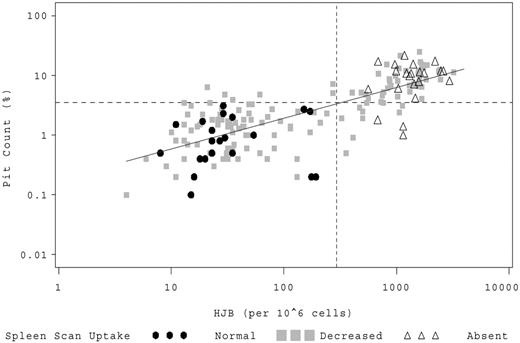
Both biomarkers, PIT and HJB, correlated well with each other (Figure 1; R = 0.77, R2 = 0.59, P < .001). Subjects with normal splenic uptake had lower values, whereas children with absent splenic function had higher values (Table 1; P < .001). The preselected biomarker thresholds appeared accurate. No child with normal splenic function had an elevation of PIT more than 3.5% or HJB more than 300/106 red blood cells (RBC). Conversely, no patient with absent splenic function had a quantitative HJB value less than 300/106 RBC, and only 4 of 26 (15%) patients with absent splenic function had PIT counts less than 3.5% (Table 1).
HJB versus PIT counts. Individual patients are represented by a symbol on the scatter plot corresponding to their qualitative LS scan result. (●●●) represents patients with normal splenic uptake; (▩▩▩), patients with splenic function that is present but decreased; and (▵▵▵), patients with absent splenic function. The dashed horizontal line denotes a log10 PIT count of 3.5%; and the vertical dashed line, log10 HJB of 300/106 RBCs.
HJB versus PIT counts. Individual patients are represented by a symbol on the scatter plot corresponding to their qualitative LS scan result. (●●●) represents patients with normal splenic uptake; (▩▩▩), patients with splenic function that is present but decreased; and (▵▵▵), patients with absent splenic function. The dashed horizontal line denotes a log10 PIT count of 3.5%; and the vertical dashed line, log10 HJB of 300/106 RBCs.
To explore the biomarker values that were most useful for differentiating between normal and absent spleen function, we performed weighted logistic regressions (Table 1). Our data suggest that a PIT of less than 1.2% best predicts normal spleen function and more than 4.5% absent function. For HJB, less than 55/106 RBC predicts normal and more than 665/106 absent spleen function.
LS scan uptake correlates with the spleen's ability to clear 1- micron particles from the blood, the size of encapsulated bacteria.12 Lack of uptake by the clinically enlarged spleen of young children with SCA was initially used to define functional asplenia.12,13 The Cooperative Study of Sickle Cell Disease reported that 20 of 64 (31%) 8- to 13-month-olds had decreased or absent uptake. No child in the Cooperative Study of Sickle Cell Disease with PIT less than 3.5% had absent uptake, but 1 of 19 (5%) with PIT more than 3.5% had normal splenic uptake (Table 1).4 The Cooperative Study of Sickle Cell Disease (CSSCD) data differed from ours in that more children had normal spleen function, possibly because our patients were somewhat older, scanned using higher doses of radionuclide and more modern equipment, with results reported by blinded readers according to predetermined criteria.
Decreasing LS scan uptake was associated with lower Hb and HbF but higher WBC, ARC (all P < .001), and ANC (P = .007). Decreasing uptake was also associated with the number of vaso-occlusive events (P < .001), dactylitis (P = .004), hospitalization other than for fever (P = .009), transfusion (P = .006), splenic sequestration (P = .012), a history of a palpable spleen (P = .012), and higher transcranial Doppler velocity (P < .001). No correlation was found with function of other organs (DTPA-GFR, kidney; or O2 saturation, lung), perhaps because the spleen is the organ first damaged in SCA and/or the sensitivity of methods for assessment of organ function in very young children is limited. There were too few episodes of bacteremia (one with absent, 3 with decreased uptake) to detect a trend.
Spleen volume was not associated with splenic function assessed by LS scan, PIT, or HJB. Larger spleen volume was associated with a history of a palpable spleen (P < .001) and decreased (but not normal or absent) uptake (P = .035). No child with a normal LS scan had a history of a palpable spleen, splenic sequestration, or transfusion, clinical events that should heighten concern that splenic function is diminished. We demonstrate that size by physical examination or ultrasound does not predict spleen function.
PIT counts ≥ 3.5% were associated with a history of transfusion (P < .001), splenic sequestration (P = .003), and a palpable spleen (P = .007). For HJB ≥ 300/106 RBC, the correlations were even stronger (P < .001 for all).
Quantifiable organ damage affects the majority of 1-year-old infants with SCA. A total of 88% of our young patients had decreased or absent splenic uptake, which correlated with older age, lower Hb and HbF, as well as higher ARC, WBC, and ANC. This is not surprising, as Hb levels less than 7 g/dL in the second year of life and higher WBCs defined SCA patients at increased risk of severe outcomes for some investigators14 but not others.15
These data reinforce the need to educate parents about the early and ongoing risk of infection with encapsulated organisms resulting from splenic dysfunction and the need to adhere to management strategies (prophylactic penicillin and prompt ER management for fever, including blood culture and empiric parenteral antibiotics) that may minimize risk.
Our data demonstrate that the biomarkers PIT and HJB correlate well with LS scan and with each other. We suggest that spleen function is a continuum better characterized by quantitative assessments than categorical LS scan interpretations. PIT counts, although quantitative and reproducible, require specialized Nomarski optics and a trained technologist. HJB quantitation requires flow cytometry. Both tests are accurate biomarkers of splenic filtrative function defined by LS scan, and both provide biologically relevant thresholds above which patients are likely to have abnormal splenic function. We conclude that the high rate of splenic dysfunction documented by 1 year of age mandates that preventive strategies be adhered to assiduously.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the BABY HUG subjects and their families for their efforts. A list of BABY HUG Clinical Sites and Investigators may be found at www.c-tasc.com/StudySites/babyhug.htm. The authors also thank Dr Beth McCarville for central review of abdominal ultrasounds and calculation of spleen volumes, Danner Li for statistical analysis, and Svetlana Avlasevich for performing the flow cytometric HJB analyses.
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (contracts N01-HB-7150 to N01-HB-07160) and supported in part by the Best Pharmaceuticals for Children Act and the National Institute of Child Health and Human Development
National Institutes of Health
Authorship
Contribution: Z.R.R., W.C.W., R.V.I., B.W.T., S.T.M., and R.E.W. designed the study; E.S.-R., S.D.D., B.F., B.L.S., and J.H.M. provided specific analytic data; Z.L. and B.W.T. provided statistical analysis; Z.R.R., R.E.W., W.C.W., S.T.M., and P.A.L. wrote the paper; and all authors provided valuable insights.
Conflict-of-interest disclosure: S.D.D. is employed by Litron Laboratories, a company that owns patents regarding flow cytometry–based scoring of micronucleated erythrocytes (ie, HJB); Litron Laboratories sells assay kits related to these patented methods that are designed for several preclinical animal models. The use described herein has not been approved or cleared by the US Food and Drug Administration. The remaining authors declare no competing financial interests.
Correspondence: Zora R. Rogers, Department of Pediatrics, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390-9063; e-mail: Zora.Rogers@UTSouthwestern.edu.