Extensive in vitro self-renewal of proerythroblasts from the earliest period of definitive erythropoiesis, as reported by England and co-investigators1 in this issue of Blood, expands the potential role of self-renewal in mammalian hematopoiesis and suggests a possible source of blood cells for clinical treatments.
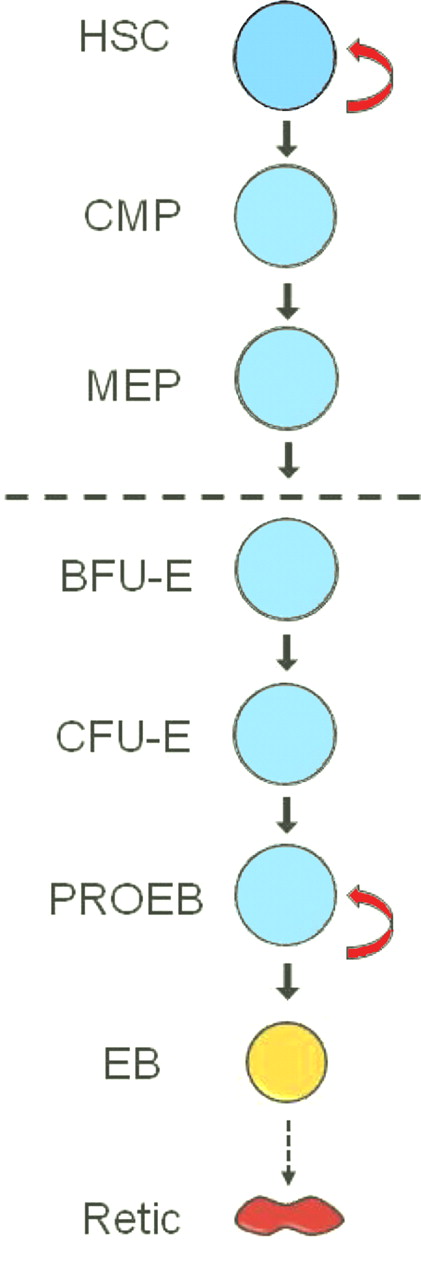
In standard hematopoietic hierarchy models, the pluripotent hematopoietic stem cell (HSC) undergoes long-term self-renewal cell divisions that create more HSCs. The HSC also commits to differentiation by cell divisions that form all lineages of lympho-hematopoietic cells. In diagrams of these models, HSC self-renewal is designated by a reflexive arrow, while another arrow leads to a sequence of bifurcating stages of hematopoietic differentiation that terminates in the mature cells of the blood and lymphoid tissues.2 The proliferative potential of progenitor cells at each successive stage of differentiation is generally assumed to be less than in the previous stage. Other than the HSC, none of the subsequent progenitors in these differentiation schemes was believed to have the potential for long-term self-renewal. Now, England et al report that, soon after the genesis of definitive erythropoiesis, the relatively late differentiation stage of the proerythroblast has the capacity for extensive self-renewal divisions in vitro.1 In the figure, this capacity for extensive self-renewal is represented by a reflexive arrow not only at the HSC stage but again at the proerythroblast stage.
In vitro differentiation of definitive erythroid cells from the mid-gestation murine fetus. Erythroid lineage differentiation begins with hematopoietic stem cell (HSC) commitment to differentiation (first solid arrow) and ends with enucleation (dashed arrow) of the erythroblast (EB) to form the reticulocyte (Retic). Stages shown are part of a continuum with multiple cell divisions at and between the successive stages. Stages below the dashed line are committed to erythroid differentiation, whereas those above the dashed line have potential to differentiate along nonerythroid lineages. The open reflexive arrows designate stages with long-term or extensive self-renewal capacity. Orange and red designate terminal differentiation stages with progressive hemoglobin accumulation. CMP indicates common myeloid progenitor; MEP, megakaryocytic-erythroid progenitor; BFU-E, burst-forming unit–erythroid; CFU-E, colony-forming unit–erythroid; and PROEB, proerythroblast.
In vitro differentiation of definitive erythroid cells from the mid-gestation murine fetus. Erythroid lineage differentiation begins with hematopoietic stem cell (HSC) commitment to differentiation (first solid arrow) and ends with enucleation (dashed arrow) of the erythroblast (EB) to form the reticulocyte (Retic). Stages shown are part of a continuum with multiple cell divisions at and between the successive stages. Stages below the dashed line are committed to erythroid differentiation, whereas those above the dashed line have potential to differentiate along nonerythroid lineages. The open reflexive arrows designate stages with long-term or extensive self-renewal capacity. Orange and red designate terminal differentiation stages with progressive hemoglobin accumulation. CMP indicates common myeloid progenitor; MEP, megakaryocytic-erythroid progenitor; BFU-E, burst-forming unit–erythroid; CFU-E, colony-forming unit–erythroid; and PROEB, proerythroblast.
The sequence of differentiation stages for the erythroid lineage includes the HSC, the common myeloid progenitor (CMP), the bipotent megakaryocytic-erythroid progenitor (MEP), and then a sequence of erythroid-restricted progenitors and morphologically distinguishable precursors, as shown below the dashed line in the figure. The erythroid-restricted progenitors were originally defined by their growth into colonies in semi-solid tissue culture, with the earlier burst-forming units–erythroid (BFU-E) having much greater proliferation potential than the subsequent colony-forming units–erythroid (CFU-E).3 Proerythroblasts bridge the differentiation gap between the functionally defined CFU-E and the morphologically defined precursor stages of basophilic through orthochromatic erythroblasts. However, proerythroblasts have a relatively nonspecific morphologic appearance, and they likely overlap functionally with mature CFU-E. In addition, CFU-E and proerythroblasts are the last stages in the erythroid differentiation sequence with kit ligand/stem cell factor (SCF) responsiveness and the earliest stages with erythropoietin (EPO) dependence.
Most previous studies of committed erythroid progenitors were performed with tissue culture media that contained EPO with or without SCF, and they confirmed the progressive loss of proliferation potential from immature BFU-E that could produce tens of thousands of terminal cells to CFU-E that divided only 3 to 6 times before reaching the terminal nonproliferative stage of orthochromatic erythroblasts. However, addition of the synthetic glucocorticoid hormone dexamethasone greatly expanded the in vitro proliferation of human erythroid progenitors that were also dependent on EPO and SCF in the culture medium.4,5 These expanded erythroid cells arose from enriched populations of BFU-E, CFU-E, and proerythroblasts,5 and they remained undifferentiated until they were cultured in medium without SCF and dexamethasone.4 When cultured without these 2 hormones, the expanded cells differentiated to orthochromatic erythroblasts and reticulocytes (EB and Retic in figure) within 4 to 5 days. Thus, EPO, SCF, and dexamethasone were required for a very large, but ultimately limited, in vitro expansion of the erythroid precursors because of cell divisions that led to self-renewal rather than differentiation. Mice with mutant glucocorticoid receptors had greatly impaired expansions of CFU-Es in response to hemolysis and hypoxia, indicating that stress erythropoiesis in vivo required glucocorticoids as well as SCF and EPO.6
Using the same 3 growth factors, England et al now demonstrate that proerythroblasts formed at the very beginning of definitive erythropoiesis in mid-gestation fetal mice can sustain in vitro self-renewal divisions much longer than proerythroblasts from older fetuses or adults.1 When they are induced to differentiate by culture in medium without dexamethasone, the proerythroblasts require only 3 to 4 cell divisions to reach the terminal erythroid precursor stage, the reticulocyte. During these terminal cell divisions, proerythroblasts undergo the major biochemical and morphologic events of terminal mammalian erythroid differentiation such as hemoglobin accumulation, reduced cellular size, and enucleation. This terminal erythroid differentiation from the proerythroblast to the reticulocyte requires approximately 2 days in mice and 4 to 5 days in humans. Thus, extensive self-renewal of proerythroblasts permits the accumulation of a very large number of cells that have not entered into terminal erythroid differentiation, but they can be induced by changes in growth factor concentrations to complete differentiation into RBCs within days.
Whether extensive self-renewal of proerythroblasts has a role in normal fetal and early postnatal life is unknown. However, body size and blood volume increase very rapidly during the perinatal period of murine development, and self-renewal of proerythroblasts may help meet the demand for increased red cell production. The extensive self-renewal of these relatively late-stage erythroid cells in vitro raises the question of whether the earlier stages of erythroid differentiation or corresponding later stages in other hematopoietic lineages may harbor similar extensive capacities to self-renew. If they do, then understanding the cellular mechanisms that permit extensive self-renewal divisions of various progenitor stages might provide sources of nonerythroid as well as erythroid blood cells for clinical use.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal