Abstract
NANOG is a master transcription factor associated with the maintenance of stem cell pluripotency. Here, we demonstrate that transcription factor NANOG is expressed in cultured endothelial cells (ECs) and in a subset of tumor cell lines. Importantly, we provide evidence that WNT3A stimulation of ECs induces the transcription of NANOG which mediates the expression of vascular endothelial growth factor receptor-2, also known as fetal liver kinase-1 (FLK1). We defined ATTA as a minimal binding site for NANOG. Accordingly, a luciferase reporter assay showed that NANOG binds to and activates 4 ATTA binding sites identified in the FLK1 promoter after WNT3A stimulation. Consistent with this data, we found that, under basal conditions and in response to WNT3A stimulation, NANOG binding to these ATTA sequences markedly induced the expression of FLK1. Thus, our data indicate an essential role in angiogenesis for NANOG binding to these 4 ATTA sites. Surprisingly, NANOG depletion not only decreased FLK1 expression but also reduced cell proliferation and angiogenesis. These findings show the necessary and sufficient role of NANOG in inducing the transcription of FLK1 to regulate the angiogenic phenotypes of ECs.
Introduction
Angiogenesis, the sprouting of new capillaries from preexisting blood vessels, is not only required for embryonic development and wound healing but also contributes to pathologic processes, including atherosclerosis, diabetic retinopathy, and tumor progression.1,2 In this regard, the activation of Wnt (Wingless) signaling in endothelial cells (ECs) has been shown to induce transcriptional events to regulate angiogenesis3-5 ; however, the underlying mechanisms of these processes are not entirely clear. Beyond binding to the cytoplasmic domain of vascular endothelial– or E-cadherin, β-catenin plays a key role in the transduction of Wnt signals by serving as a coactivator for the transcription factor T-cell factor/lymphocyte enhancer binding factor (TCF/LEF-1).6,7 The ligation of Wnt to the Frizzled receptor induces the inhibition of glycogen synthase kinase-3β that results in decreased phosphorylation of β-catenin, thereby reduced proteolysis and degradation.5-7 Stabilized β-catenin translocates into the nucleus to associate with transcription factors of the TCF/LEF-1 family that can transactivate genes containing TCF/LEF-1 binding sequences.5-7 Recent gene expression profiling has identified a role for Wnt signaling in EC commitment8 and in control of vasculo-angiogenic aspects of embryonic development.9-15
NANOG is a divergent homeobox transcription factor expressed in germ cells and pluripotent stem cells that is critical for morphogenesis and embryonic development.16-19 Mouse embryos lacking nanog die between days embryonic (E) 3.5 and E5.5, before any vasculature has developed.20,21 Nanog overexpression renders mouse embryonic stem (ES) cells independent of leukemia inhibitory factor/signal transducer and activator of transcription-3 stimulation for self-renewal.17-19 Analyses of the promoter and enhancer regions of the human NANOG gene have shown binding sites for several key transcription factors, including OCT3/4, SOX2, BRACHYURY, KLF4, and NANOG itself.18-21 However, if these genes are expressed in adult tissues to give rise to stem or progenitor cells during pathophysiologic process is not known.
NANOG mRNA transcripts are present in the aorta-gonad-mesonephros, a region of the embryonic mesoderm that gives rise to hemangioblastic EC precursors.22 Expression of NANOG is also observed in endometriosis samples.23 Therefore, a key question arises whether NANOG is involved in the regulation of angiogenesis. The absence of the fetal liver kinase (flk1) gene is incompatible with life because these mutant embryos lack ECs and die in utero due to defects in hematopoiesis and the formation of blood vessels.24 The observations that WNT3A induces the expression of OCT4 and NANOG in progenitor and primitive cells,25-28 the proliferation of ECs, and tubule formation29 suggest a potential role for NANOG in angiogenesis. Thus, here we tested the hypothesis that NANOG plays a central role in regulating the angiogenic phenotype of ECs through the transcriptional activation of the FLK1 gene.
Methods
Antibodies and reagents
The mouse anti–human NANOG monoclonal antibody (mAb) and mouse anti–human KLF4 mAb were purchased from Abnova. Rabbit anti–mouse Nanog was purchased from Bethyl Laboratories. Rabbit anti-FLK1, rabbit anti–human/mouse/rat FLK1, mouse anti–human β-catenin (E-5), mouse anti–glyceraldehyde-3-phosphate dehydrogenase, rabbit anti–vascular endothelial-cadherin, donkey anti–mouse immunoglobulin G (IgG)–horseradish peroxidase, and donkey anti–rabbit IgG–horseradish peroxidase, and small interfering RNAs (siRNAs; modified 25-mer duplexes, stable in serum-containing media) were purchased from Santa Cruz Biotechnology. Premade control nonsilencing and NANOG shRNA retroviral particles were purchased from Open Biosystems Inc. Human recombinant WNT3A and growth factor–reduced Matrigel were purchased from R&D Systems.
Whole-mount immunohistochemical staining
Mouse embryos at day E14.5 were collected and fixed immediately in freshly prepared 4% para-formaldehyde in phosphate-buffered saline (PBS) for 1 hour. After a quick wash in PBS, pH 7.4, embryos were incubated with 5% H2O2 for 4 hours at room temperature and permeabilized with 0.5% Triton X-100 overnight at 4°C. Subsequently, embryos were immersed in blocking solution, PBS-MT (PBS containing 2% Carnation dry milk and 0.1% Triton X-100) for 2 hours at room temperature, then incubated for 2 days in anti–mouse Nanog Alexa Fluor 647 antibody (eBioscience) diluted 1:100 in blocking solution at 4°C. Then embryos were mounted in 50% glycerol, and expression of Nanog was photographed with the use of a Zeiss stereo microscope (Model Discovery.V12) with the use of an Axiocam digital camera controlled by AxioVision software Version 4.6. AxioVision digital images were then saved as TIFFs using Adobe Photoshop CS. Multiple images were assembled using QuarkXpress 8.0 software and labeled, and final images were saved as EPS documents.
Cell culture, siRNA transfection, and Western blot analysis
Human umbilical vein ECs (HUVECs), human dermal microvascular ECs (HdMVECs), and human lung microvascular ECs (LMVECs) were purchased from Lonza and cultured with the use of an EndoGRO-VEGF (vascular endothelial growth factor) Complete Media Kit (Millipore) as previously described.30-32 Human ES cells (hESCs) were obtained from WiCell (H1 line; Wicell Institute USA) and were cultured according Ludwig et al.33 Experiments with hESCs were conducted according to guidelines and regulations of the University of Illinois at Chicago Embryonic Stem Cell Research Oversight Committee. Transient transfection, siRNA (chemically synthesized), and short hairpin RNA (shRNA; retrovirus)–mediated knockdown and knockdown efficiency were evaluated as described.31,32 For Western blot (WB) analysis, ECs were solubilized in cell lysis buffer containing 20mM Tris (tris(hydroxymethyl)aminomethane; pH 7.5), 1% Triton X-100, 0.2% NP-40, 150mM NaCl, and protease inhibitors as previously described.30-32 Sodium dodecyl sulfate–polyacrylamide gel electrophoresis, membrane transfer, antibody dilution, incubation, and WB analysis were carried out as previously described.30-32
RNA extraction, RT-PCR, qRT-PCR, 5′-bromo-2′-deoxyuridine incorporation assay, and microscopy
RNA extraction and reverse transcription–polymerase chain reaction (RT-PCR) methods have been previously described.30-32 The following primers were obtained from IDT DNA Technologies: NANOG (NM_024865), forward primer, 5′-CCAGCCTTTACTCTTCCTACCA-3′, and reverse primer, 5′-GCTGATTAGGCTCCAACCATAC-3′ (product size, 570 bp); FLK1 (also known as VEGFR2/KDR; NM_002253), forward primer, 5′-TTCTGGACTCTCTCTGCCTACC-3′, and reverse primer, 5′-AGAACCATACCACTGTCCGTCT-3′ (product size, 225 bp); and GAPDH (NM_002046), forward primer, 5′-AGAACATCATCCCTGCCTCTACT-3′, and reverse primer, 5′-TCTCTCTTCCTCTTGTGCTCTTG-3′ (product size, 444 bp). These experiments were carried out as described previously.34 5′-Bromo-2′-deoxyuridine (BrdU) incorporation into HUVECs as a measurement of cell proliferation was carried out as described previously.30 Images were captured using a Zeiss microscope Axiovert 40 C, 20× objectives, saved as TIFF documents, and converted to EPS format using Adobe Photoshop CS. To assemble multiple images, QuarkXpress 8.0 software was used. Experiments were carried out ≥ 3 times.
Human FLK1-luciferase reporter constructs and luciferase assays, chromatin immunoprecipitation, and electrophoretic mobility shift assays
These experiments were carried out as described previously.34 For the reporter-luciferase constructs, wild-type and mutant constructs of the human FLK1 promoter were subcloned into the pGL3-Basic-Luciferase vector between the KpnI and HindIII restriction sites, and the clones were verified by DNA sequencing (Genscript). The transfection of constructs and luciferase assays has been described previously.30-32 For each experiment, the total concentration of the plasmids used for transfection was adjusted to an equivalent final concentration. The luciferase activity in cell lysates were normalized to β-galactosidase activity (to correct for transfection efficiency) by cotransfecting trace amounts of the pCMV-β-galactosidase plasmid in all experiments.30-32 For the chromatin immunoprecipitation (ChIP) assays, ECs were grown in complete medium and left untreated (control) or treated with WNT3A (50 ng/mL) for 72 hours. For the electrophoretic mobility shift assays (EMSAs), nuclear extracts from mouse brain tissue were prepared with the use of NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce) according to the manufacturer's instructions. Oligonucleotides for the EMSA of the FLK1 promoter were obtained from IDT DNA Technologies and labeled with the Biotin 3′ End DNA Labeling Kit (Pierce), and the EMSA reactions were carried out as described previously.34
Formation of branching point structures
Branching point structure formation assay has been described previously.31,32 In brief, human LMVECs were detached from flasks with 2mM ethylenediaminetetraacetic acid, pH 7.4, washed with PBS, resuspended in defined medium (Endothelial Cell Basal Medium 2 containing WNT3A and VEGF165). Control LMVECs (1 × 105; at ∼ 60% density) and were left untreated or transfected with either control shRNA retrovirus or NANOG shRNA retrovirus + FLK1 plasmid cDNA (for rescue experiment). After ∼ 16 hours, LMVECs were plated onto polymerized 3-dimensional type I collagen gel and incubated at 37°C for 12 hours. The unattached LMVECs were removed and then overlaid with a second layer of type I collagen. After 36 hours, the cells were washed with PBS and photographed under a phase-contrast microscope (Zeiss Axiovert 40 C, 20× objectives), using a Canon Powershot digital camera. Digital images were saved as TIFF documents using Adobe Photoshop CS. Multiple images were assembled using QuarkXpress 8.0 software and labeled, and final images were saved as EPS documents. For quantification ≥ 10 random fields were examined. Experiments were repeated 3 times.
Matrigel plug assay
For the Matrigel plug assays, 3-month-old athymic nude mice (25-30 g body weight; The Jackson Laboratory) were used. The mice were housed under pathogen-free conditions at the University of Illinois Animal Care Vivarium and treated humanely in accordance with institutional guidelines. Growth factor–reduced Matrigel (250 μL) + WNT3A (50 ng/mL) containing 105 HUVECs with or without NANOG shRNA or with NANOG shRNA + FLK1 cDNA were injected subcutaneously in the midventral abdominal region of nude mice, allowed to solidify, and monitored after 24 and 48 hours to assess the wound. After 7 days, the Matrigel plugs were collected and separated from the abdominal muscle, washed with PBS, fixed in 10% formalin, and embedded in paraffin. The experiment was repeated twice. Five- to 6-μm serial sections were prepared and stained with hematoxylin and eosin. To visualize the formation of capillaries, a Zeiss Axiovert 40 phase contrast microscope was used. At least 5 Matrigel plugs were used for each group. For quantification, 10 random fields were selected and examined at ×200 magnification in 5 different sections of each of 5 Matrigel plugs. Images were captured with the use of a digital camera and saved as TIFF files. Figures were assembled with the use of QuarkExpress 8.0 software (Quark Inc), then images were saved as an EPS document.
Statistics
For statistical analysis, GraphPad Prizm 5.0 software was used (GraphPad Software). An unpaired Student t test was used for comparisons between 2 groups. Analysis of variance followed by an unpaired Student t test was used for multiple comparisons. The level of significance was set at P < .05.
Results
WNT3A induces NANOG protein expression in ECs
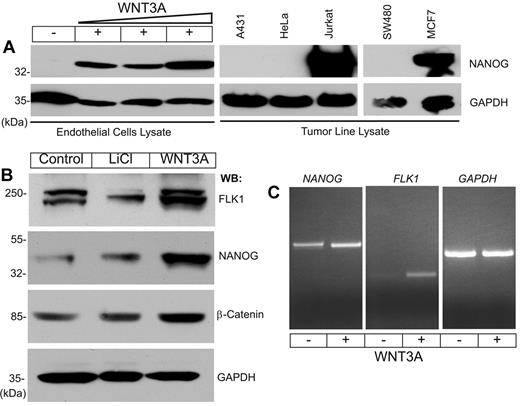
To address the relationship between WNT signaling and NANOG in EC proliferation and angiogenesis, primary human LMVECs, HdMVECs, HUVECs, and human lung fibroblast (MRC-5) cells were studied (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The expression of NANOG increased in a dose-dependent manner in WNT3A-stimulated cells compared with the low or undetectable levels observed in controls (Figure 1A). Among the cell lines tested, NANOG was constitutively expressed in proliferating Jurkat (T-cell), and MCF7 (breast carcinoma) cells, but it was not detectable in A431 (epidermoid carcinoma), HeLa (cervical carcinoma), or SW480 (colon tumor) cells (Figure 1A). To confirm the responsiveness of HUVECs to WNT3A stimulation, we determined the expression of β-catenin, NANOG, and FLK1 proteins in ECs grown in the presence of serum and growth factors. We included LiCl, an inhibitor of glycogen synthase kinase-3β, to monitor the relative stability of β-catenin in these cells. Given that the ECs were not starved of growth factors for this experiment, we observed basal expression of β-catenin, NANOG, and FLK1 proteins in control cells and increased expression of these proteins in response to both LiCl and WNT3A stimulation (Figure 1B). We also confirmed the expression of NANOG and FLK1 transcripts by RT-PCR (Figure 1C). These results indicate that NANOG protein synthesis may be undetectable in ECs starved of growth factors and serum, but it is possibly induced by serum factors and enhanced after WNT3A stimulation. Furthermore, quantitative PCR assay showed increased NANOG and FLK1 transcripts in HUVECs in response to WNT3A in a dose-dependent manner (supplemental Figure 2). Human ES cells (hESC-H1 line) basally expressed NANOG, SOX2, and OCT4 transcripts close to 60- to 90-fold (supplemental Figure 3), in contrast to HUVECs that expressed 15-fold NANOG transcript only in response to WNT3A stimulation (supplemental Figure 3).
NANOG expression in HUVECs and tumor cell lines. (A) HUVECs were starved of growth factors and serum for 14 hours, then left untreated or treated with increasing concentrations of WNT3A (10, 20, or 30 ng/mL) for 6 hours. Total protein lysates prepared from tumor cell lines were subjected to WB analysis with the indicated antibodies. (B) Unstarved HUVECs were left untreated or treated with LiCl (20 ng/mL) or WNT3A (50 ng/mL) for 6 hours. Total cell lysates were analyzed by WB analysis with the indicated antibodies. Anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to determine equal protein loading. (C) Total RNA prepared from HUVECs left untreated or treated with WNT3A was subjected to RT-PCR for NANOG, FLK1, and GAPDH. Each experiment was repeated ≥ 3 times.
NANOG expression in HUVECs and tumor cell lines. (A) HUVECs were starved of growth factors and serum for 14 hours, then left untreated or treated with increasing concentrations of WNT3A (10, 20, or 30 ng/mL) for 6 hours. Total protein lysates prepared from tumor cell lines were subjected to WB analysis with the indicated antibodies. (B) Unstarved HUVECs were left untreated or treated with LiCl (20 ng/mL) or WNT3A (50 ng/mL) for 6 hours. Total cell lysates were analyzed by WB analysis with the indicated antibodies. Anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to determine equal protein loading. (C) Total RNA prepared from HUVECs left untreated or treated with WNT3A was subjected to RT-PCR for NANOG, FLK1, and GAPDH. Each experiment was repeated ≥ 3 times.
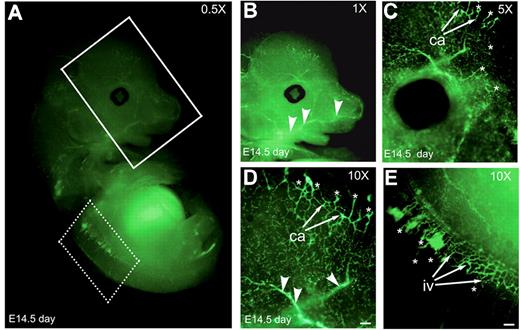
To examine if Nanog is expressed in developing blood vessels, we fixed and stained E14.5-day-old whole-mount embryo with anti-Nanog antibody (Figure 2A). Developing capillaries and intersomitic vessels were positively stained with anti–Nanog mAb. Anti–Nanog mAb stained sprouting ECs (Figure 2B-D asterisks). Anti-Nanog mAb detected both large- (arrowheads) and small-caliber (Figure 2B-D arrows) neovessels.
Embryo at 14.5 days shows developing blood vessels. (A) Whole-mount Nanog staining of an E14.5 embryo. The vasculature of the head and brain (solid line boxed area) and intersomitic vessels (dotted line boxed area) at indicated magnification. (B-D) Vasculature of the head and brain regions at higher magnifications. (E) Nanog is expressed at the intersomitic vessels as shown in higher magnification. Ca indicates, capillaries; iv, intersomitic vessels; asterisks, sprouting ECs; arrowheads, large-caliber neovessels. Scale bar, 20 μm. Results are representative of 3 experiments with similar results.
Embryo at 14.5 days shows developing blood vessels. (A) Whole-mount Nanog staining of an E14.5 embryo. The vasculature of the head and brain (solid line boxed area) and intersomitic vessels (dotted line boxed area) at indicated magnification. (B-D) Vasculature of the head and brain regions at higher magnifications. (E) Nanog is expressed at the intersomitic vessels as shown in higher magnification. Ca indicates, capillaries; iv, intersomitic vessels; asterisks, sprouting ECs; arrowheads, large-caliber neovessels. Scale bar, 20 μm. Results are representative of 3 experiments with similar results.
NANOG binds to and activates the FLK1 promoter in response to WNT3A
We next evaluated the interaction of NANOG with the FLK1 promoter. Analysis of the human FLK1 promoter from −1460 to 1 relative to the transcription start site identified 8 putative NANOG binding sites (ATTA). The human FLK1 promoter sequence is shown (supplemental Figure 4), and putative binding sites are indicated in red.
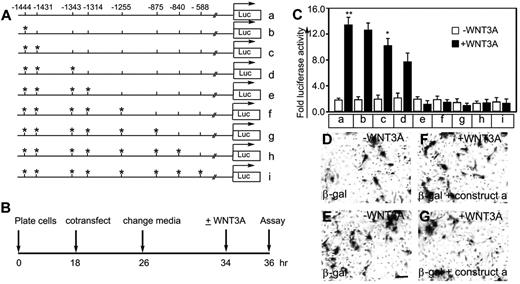
To test the hypothesis that WNT3A-induced activation of NANOG is required for FLK1 promoter activity, we generated FLK1 promoter-luciferase reporter constructs. The wild-type construct (Figure 3A row a) contained all 8 putative NANOG-binding sites, whereas the mutant constructs (Figure 3A rows b-i) carried point mutations (ATTA to AaTA) in the indicated sites (Figure 3A asterisks). To determine promoter-reporter activity, HUVECs were cotransfected with a trace amount of β-galactosidase and either a wild-type or mutant construct. The timeline of the experiment is shown (Figure 3B). Basal luciferase activity was 1- to 2-fold in the untreated (WNT3A) ECs (Figure 3C). The WNT3A-treated ECs cotransfected with the wild-type (Figure 3C construct a) or mutant (Figure 3C constructs b-d) constructs showed increased luciferase activity by 6.6-, 5.5-, 4.5-, and 4-fold, respectively (Figure 3C). In contrast, there was no induction of luciferase activity in the ECs transfected with mutant constructs (Figure 3C constructs e-i). Mutations at positions −1444, −1431, −1343, and −1314 (Figure 3C construct e) reduced the luciferase activity to baseline levels. The transfection efficiency in control ECs treated with or without WNT3A was monitored by staining with X-gal (Figure 3D-G). These results show that the ATTA sites at −1444, −1431, −1343, and −1314 upstream of the transcription start site are responsive to WNT3A stimulation and are probably bound by NANOG.
A human FLK1 promoter-luciferase reporter assay identifies 4 potential NANOG binding sites. (A) Wild-type and point-mutant human FLK1 promoter constructs (a-i). Asterisks indicate point mutations of ATTA to AaTA. (B) Timeline of the transfection experiments and luciferase assay. (C) The indicated wild-type and point-mutant FLK1 promoter constructs driving the luciferase reporter together with a trace amount of β-galactosidase were transiently transfected into HUVECs, which were left untreated (control) or treated with WNT3A (50 ng/mL) (no serum or growth factor). Fold luciferase activity is shown as the mean ± SEM; n = 5-10 from 3-4 independent experiments; *P < .05 and **P < .01 compared with control (without WNT3A treatment). (D-G) Transfection efficiency was determined by staining the transfected HUVECs with X-gal. Magnification, ×100; scale bar, 50 μm. Images were recorded through a Zeiss Axiovert 40 C microscope, 20× objectives, using a Canon Powershot digital camera. Digital images were saved as TIFF documents using Adobe Photoshop CS. Multiple images were assembled using QuarkXpress 8.0 software and labeled, and final images were saved as EPS documents. Experiments were carried out ≥ 3 times.
A human FLK1 promoter-luciferase reporter assay identifies 4 potential NANOG binding sites. (A) Wild-type and point-mutant human FLK1 promoter constructs (a-i). Asterisks indicate point mutations of ATTA to AaTA. (B) Timeline of the transfection experiments and luciferase assay. (C) The indicated wild-type and point-mutant FLK1 promoter constructs driving the luciferase reporter together with a trace amount of β-galactosidase were transiently transfected into HUVECs, which were left untreated (control) or treated with WNT3A (50 ng/mL) (no serum or growth factor). Fold luciferase activity is shown as the mean ± SEM; n = 5-10 from 3-4 independent experiments; *P < .05 and **P < .01 compared with control (without WNT3A treatment). (D-G) Transfection efficiency was determined by staining the transfected HUVECs with X-gal. Magnification, ×100; scale bar, 50 μm. Images were recorded through a Zeiss Axiovert 40 C microscope, 20× objectives, using a Canon Powershot digital camera. Digital images were saved as TIFF documents using Adobe Photoshop CS. Multiple images were assembled using QuarkXpress 8.0 software and labeled, and final images were saved as EPS documents. Experiments were carried out ≥ 3 times.
NANOG and KLF4 bind to the NANOG promoter/enhancer, and NANOG binds to the FLK1 promoter
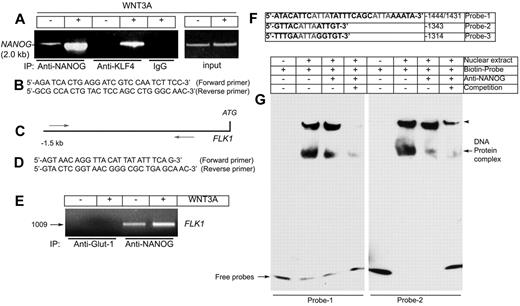
Given that the ECs expressed NANOG in response to WNT3A stimulation, we next investigated whether NANOG protein could bind to the NANOG promoter/enhancer region. Binding sites for NANOG and Krüppel-like factor-4 (KLF4) have been reported within the NANOG promoter/enhancer,35-37 and we included KLF4 as a positive control. To test whether NANOG and KLF4 both bind to the NANOG promoter, primers were designed to amplify this region and were used in a ChIP assay. A genomic sequence corresponding to Homo sapiens NANOG was used as a template. Analysis of the PCR products showed that both NANOG and KLF4 bound to the 2.0-kb NANOG promoter sequence after WNT3A stimulation (Figure 4A). The minimum basal binding levels were detected in control HUVECs and were significantly increased after WNT3A stimulation (Figure 4A second and fourth lanes). Importantly, the immunoprecipitation was specific for NANOG and KLF4 because we did not detect the NANOG promoter when a nonspecific antibody was used for immunoprecipitation (Figure 4A IP:IgG last lane). To determine whether NANOG binds to its putative binding sites in the FLK1 promoter, we examined the association of NANOG with the FLK1 promoter with the use of a ChIP assay (Figure 4C-E). We designed PCR primers to amplify the NANOG binding sites in the human FLK1 promoter as shown (Figure 4C-D). There was no detectable binding in a control anti–Glut-1 immunoprecipitation (Figure 4E first and second lanes). Minimal basal binding of NANOG to the FLK1 promoter was observed in control ECs but was significantly increased after WNT3A stimulation (Figure 4E third and fourth lanes). Collectively, these data suggest that WNT3A induces the expression of NANOG and KLF4, both of which bind to the NANOG promoter, whereas NANOG binds to the FLK1 promoter.
NANOG and KLF4 both bind to the NANOG promoter/enhancer and NANOG binds to the FLK1 promoter/enhancer in response to WNT3A. (A) HUVECs were starved of growth factors and serum, then left untreated or treated for 120 minutes with WNT3A (50 ng/mL). ChIP was performed with antibodies (Abs) specific for NANOG and KLF4, in addition to a nonspecific anti-rabbit IgG, as indicated (left). The left panel shows the PCR products (2.0 kb) from the human NANOG promoter/enhancer with the use of input chromatin prepared from HUVECs treated with or without WNT3A. (B) The primers used for amplification of the human NANOG promoter/enhancer region. (C) The relative positions of the primers used for amplification of the human FLK1 promoter region flanking the 8 putative NANOG binding sites. (D) Primers used for amplification of the human FLK1 promoter. (E) Interaction of NANOG with the FLK1 promoter. PCR product of the FLK1 promoter/enhancer (1.0 kb). (F) Biotinylated probes used for the EMSA. (G) Representative images of the EMSA experiment. Biotin-labeled oligonucleotides (probe 1 and probe 2) containing the sequences of the putative NANOG binding sites from the FLK1 promoter were incubated with nuclear extracts. The supershift was carried out by preincubation of the nuclear extracts with an anti-NANOG antibody. Note: Both monomeric and dimeric forms of NANOG interacted with the biotinylated probes. Arrowheads indicate the dimeric forms of NANOG in a native gel. Experiments were carried out ≥ 5 times.
NANOG and KLF4 both bind to the NANOG promoter/enhancer and NANOG binds to the FLK1 promoter/enhancer in response to WNT3A. (A) HUVECs were starved of growth factors and serum, then left untreated or treated for 120 minutes with WNT3A (50 ng/mL). ChIP was performed with antibodies (Abs) specific for NANOG and KLF4, in addition to a nonspecific anti-rabbit IgG, as indicated (left). The left panel shows the PCR products (2.0 kb) from the human NANOG promoter/enhancer with the use of input chromatin prepared from HUVECs treated with or without WNT3A. (B) The primers used for amplification of the human NANOG promoter/enhancer region. (C) The relative positions of the primers used for amplification of the human FLK1 promoter region flanking the 8 putative NANOG binding sites. (D) Primers used for amplification of the human FLK1 promoter. (E) Interaction of NANOG with the FLK1 promoter. PCR product of the FLK1 promoter/enhancer (1.0 kb). (F) Biotinylated probes used for the EMSA. (G) Representative images of the EMSA experiment. Biotin-labeled oligonucleotides (probe 1 and probe 2) containing the sequences of the putative NANOG binding sites from the FLK1 promoter were incubated with nuclear extracts. The supershift was carried out by preincubation of the nuclear extracts with an anti-NANOG antibody. Note: Both monomeric and dimeric forms of NANOG interacted with the biotinylated probes. Arrowheads indicate the dimeric forms of NANOG in a native gel. Experiments were carried out ≥ 5 times.
To determine the mechanism of NANOG binding to the FLK1 promoter, 3 biotinylated primers flanking the −1444 and −1431 (probe 1), −1343 (probe 2), and −1314 (probe 3) ATTA sites were synthesized (Figure 4F). Probe 1 contained 2 ATTA sites in tandem. Using these probes, we performed EMSAs to determine the interaction of NANOG with the FLK1 promoter (Figure 4G). We observed an interaction of NANOG with FLK1 promoter sequence elements flanking the −1444/1431, −1343 (Figure 4G), and −1314 sites (data not shown). In contrast, incubation with an unlabeled oligonucleotide decreased the specific binding of NANOG to the biotin-labeled FLK1 promoter (Figure 4G last lanes). These findings suggest that NANOG is capable of binding to the FLK1 promoter at the studied sites.
NANOG regulates EC proliferation
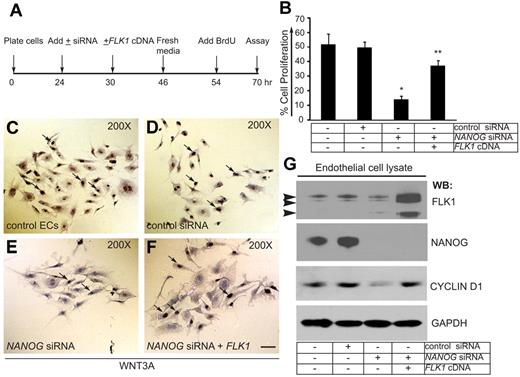
In pilot experiments, we detected expression of NANOG transcript in response to WNT3A stimulation at 60 minutes, whereas expression of NANOG and FLK1 proteins were detectable as early as 6 hours and as late as 72 hours in response to 1 dose of WNT3A (50 ng/mL) treatment. After 72 hours ECs were no longer dividing, yet the expression of NANOG and FLK1 were persistent and stable (data not shown). To determine the physiologic significance of NANOG expression and its interaction with the FLK1 promoter, we evaluated the effects of NANOG knockdown on WNT3A induced HUVECs proliferation in vitro (Figure 5). The timeline of EC proliferation assay is shown in Figure 5A. Control ECs and ECs transfected with the control siRNA showed ∼ 50% BrdU-positive cells (Figure 5B). In contrast, depletion of NANOG resulted in only 12% BrdU-positive cells (Figure 5B). To determine whether expression of a FLK1 cDNA could reverse the effect of NANOG depletion, we measured BrdU incorporation in these cells. Partial restoration of the ability of ECs to incorporate BrdU was observed after FLK1 cDNA transfection in NANOG-depleted cells (Figure 5B). Representative images of the BrdU incorporation assay are shown (Figure 5C-F). The efficiency of NANOG depletion (100%) and expression of the FLK1 cDNA were determined by WB analysis (Figure 5G). Transfection of FLK1-cDNA into NANOG-depleted ECs normalized the expression of CYCLIN-D1 (Figure 5G). This data show that FLK1 is a downstream target of NANOG.
NANOG knockdown decreases WNT3A-induced HUVEC proliferation. (A) Timeline of knockdown, FLK1 cDNA transfection (rescue), and the BrdU assay. (B) Quantification of BrdU incorporation in control HUVECs and HUVECs treated with control siRNA, NANOG siRNA, or NANOG siRNA + FLK1 cDNA (siRNAs used for this experiments were chemically synthesized) (knockdowns were performed in OPTI-MEM media + WNT3A). The cells were incubated with BrdU (1.0 μg/mL) for 16 hours in media containing WNT3A (without serum), then fixed, stained, and quantified. For quantification, ≥ 10 random fields were selected from each coverslip and examined under ×200 magnification. The data represent the mean ± SEM; n = 6-10 from 5-7 independent experiments; *P < .05 and **P < .01 compared with control. (C-F) Representative images of the cell proliferation assays. White arrows indicate BrdU-positive cells. (G) The efficiency of NANOG knockdown and FLK1 cDNA reexpression was determined by WB analysis with the indicated antibodies. The NANOG protein appeared as a 45- to 50-kDa polypeptide. Arrowheads indicate 230-, 200-, and 150-kDa anti-FLK1 antibody immunoreactive bands. Anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to determine equal protein loading. Experiments were performed ≥ 3 times.
NANOG knockdown decreases WNT3A-induced HUVEC proliferation. (A) Timeline of knockdown, FLK1 cDNA transfection (rescue), and the BrdU assay. (B) Quantification of BrdU incorporation in control HUVECs and HUVECs treated with control siRNA, NANOG siRNA, or NANOG siRNA + FLK1 cDNA (siRNAs used for this experiments were chemically synthesized) (knockdowns were performed in OPTI-MEM media + WNT3A). The cells were incubated with BrdU (1.0 μg/mL) for 16 hours in media containing WNT3A (without serum), then fixed, stained, and quantified. For quantification, ≥ 10 random fields were selected from each coverslip and examined under ×200 magnification. The data represent the mean ± SEM; n = 6-10 from 5-7 independent experiments; *P < .05 and **P < .01 compared with control. (C-F) Representative images of the cell proliferation assays. White arrows indicate BrdU-positive cells. (G) The efficiency of NANOG knockdown and FLK1 cDNA reexpression was determined by WB analysis with the indicated antibodies. The NANOG protein appeared as a 45- to 50-kDa polypeptide. Arrowheads indicate 230-, 200-, and 150-kDa anti-FLK1 antibody immunoreactive bands. Anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to determine equal protein loading. Experiments were performed ≥ 3 times.
NANOG regulates formation of branching point structures
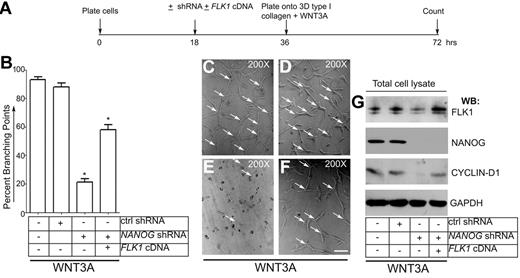
To address the role of NANOG in mediating the expression of FLK1 in angiogenesis, we knocked down the NANOG gene and assayed the formation of LMVECs branching point structures. Human LMVECs were left untreated (control LMVECs) or infected with control shRNA or NANOG-shRNA (retrovirus). To examine whether reexpression of FLK1 reverses the effect of loss of NANOG, NANOG-depleted LMVECs were infected with plasmid encoding the full-length human FLK1-cDNA. To induce formation branching point structures, infected cells were plated on 3-dimensional type I collagen gels (Figure 6A-G). This experiment was made with media containing WNT3A and VEGF without serum. The formation of a mosaic of EC interconnections called branching point structures were counted at the end of the 36-hour period (Figure 6B). Representative images are shown in Figure 6C-F. Control LMVECs and LMVECs infected with control shRNA showed comparable number of branching point structures. However, we found decreased branching points in NANOG-depleted LMVECs. Importantly, the formation of branching point structures was significantly restored in LMVECs transfected with plasmid encoding full-length FLK1-cDNA (Figure 6B,E-F). Transfection of FLK1-cDNA into NANOG-depleted LMVECs also restored the CYCLIN-D1 expression to basal level (Figure 5F). The extent of NANOG depletion (100%) and expression of the FLK1 were determined by WB analysis (Figure 6G). We noted that the transfection of FLK1-cDNA into NANOG-depleted LMVECs significantly restored the effects of loss of NANOG.
NANOG-knockdown disrupts formation of branching point structures. (A) Timeline of experiment. (B) Quantification of branching points. To deplete endogenous NANOG, human LMVECs at 50% density (2 × 106) were either left untreated or infected with control shRNA or NANOG shRNA (retrovirus) for 16 hours (in presence of serum and growth factors). For rescue experiment, FLK1-cDNA plasmid (1.5 μg/2 × 106 cells) was included along with NANOG-shRNA. Cells were detached with 2mM ethylenediaminetetraacetic acid, pH 7.4, washed with PBS, then plated onto 3-dimensional type I collagen matrix + WNT3A (50 ng/mL) (no serum and growth factors added from this time point). After 36 hours, the number of branching points were counted. Data are expressed as the percentage of branching points; n = 5; *P < .05 compared with control or as indicated. (C-F) Representative images of branching point structures. Experiments were repeated 3 times with the use of triplicate wells. Magnification, ×200. Scale bar, 200μm. Arrows indicate branching points. (G) Whole-cell lysates were subjected to WB analyses with the antibodies indicated. All blots shown are representative of 3 independent experiments.
NANOG-knockdown disrupts formation of branching point structures. (A) Timeline of experiment. (B) Quantification of branching points. To deplete endogenous NANOG, human LMVECs at 50% density (2 × 106) were either left untreated or infected with control shRNA or NANOG shRNA (retrovirus) for 16 hours (in presence of serum and growth factors). For rescue experiment, FLK1-cDNA plasmid (1.5 μg/2 × 106 cells) was included along with NANOG-shRNA. Cells were detached with 2mM ethylenediaminetetraacetic acid, pH 7.4, washed with PBS, then plated onto 3-dimensional type I collagen matrix + WNT3A (50 ng/mL) (no serum and growth factors added from this time point). After 36 hours, the number of branching points were counted. Data are expressed as the percentage of branching points; n = 5; *P < .05 compared with control or as indicated. (C-F) Representative images of branching point structures. Experiments were repeated 3 times with the use of triplicate wells. Magnification, ×200. Scale bar, 200μm. Arrows indicate branching points. (G) Whole-cell lysates were subjected to WB analyses with the antibodies indicated. All blots shown are representative of 3 independent experiments.
NANOG knockdown decreases angiogenesis in Matrigel plug assay in vivo
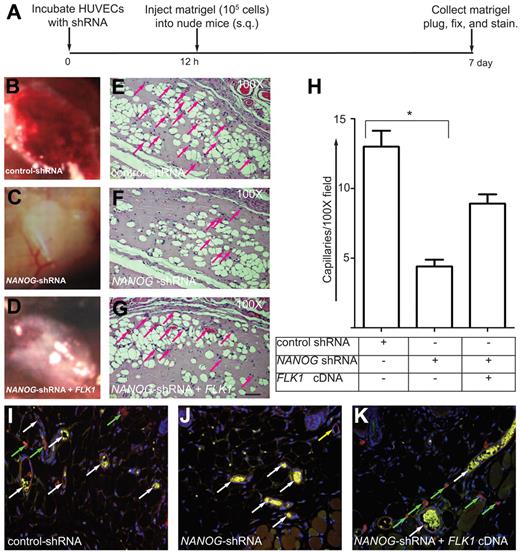
To measure the contribution of NANOG to angiogenesis, we used a Matrigel plug assay (Figure 7A-K). Figure 7B shows control HUVECs induced highly vascularized Matrigel plugs, whereas the NANOG depletion reduced this effect (Figure 7C), and expression of FLK1-cDNA only partially restored the effect of depletion of NANOG (Figure 7D). The formation of neovessels in Matrigel plug sections was determined by the presence of red blood cells (Figure 7E-G; supplemental Figure 5). Only those neovessels that were filled with red blood cells (arrows) were counted as productive neovessels (Figure 7E-G red arrows; supplemental Figure 5). As expected, the control HUVECs showed a brisk angiogenic response in the Matrigel plugs (> 12-fold), and this response was strongly inhibited by NANOG knockdown (< 5-fold; second histogram bar; Figure 7H). Expression of human FLK1 cDNA in the NANOG-depleted cells partially reversed the effect of NANOG depletion (8-fold; third bar; Figure 7G-H). Next, thin Matrigel cryosections incubated with anti–human von Willebrand factor antibody showed highly specific red fluorescent HUVECs (Figure 7I-K), derived capillaries with discrete intracellular clusters of von Willebrand factor (Figure 7I-K). The HUVECs had aligned and formed both large-and small-caliber neovessels. Intense autofluorescent red blood cells served as a marker of perfusion (Figure 7I-K). Anti–mouse CD31 staining in Matrigel showed many migrating cells, some of them organized into bundles and neovessels made of HUVECs and mouse ECs (Figure 7I-K white arrows). Neovessels formed entirely of HUVECs are stained red (Figure 7J yellow arrow). We also observed that a few control HUVECs as well as NANOG-depleted HUVECs that did not form blood vessels remained scattered outside of neovessels (Figure 7I-K green arrows). These data show that NANOG regulates angiogenesis in vivo by inducing FLK1-mediated signaling and EC proliferation.
NANOG knockdown decreases angiogenesis in Matrigel plug assays. (A) Timeline of the Matrigel plug assay. (B-D) HUVECs were incubated with the indicated shRNAs (retrovirus) for 10 hours (in presence of serum and growth factors), after which time the cells (105) were mixed with growth facto–reduced Matrigel (250 μL) plus WNT3A (50 ng/mL) and injected subcutaneously into athymic nude mice (n = 5). After 7 days, the Matrigel plugs were collected, embedded in paraffin, sectioned (4-5 μm), and stained with hematoxylin and eosin. Pictures of Matrigel plugs were taken using a Canon Powershot A640 digital camera controlled by Zoom Browser Ex software 5.7. Digital images were then saved as EPS documents using Adobe Photoshop CS. Multiple images were assembled using QuarkXpress 8.0 software and labeled, and final images were saved as EPS documents. (E-G) Note the prominent microvessels in the control shRNA (noninterfering shRNA retrovirus) plugs and the reduced number of capillaries in the NANOG knockdown plugs. FLK1 cDNA expression partially compensated for the loss of NANOG. Scale bar, 40 μm. (H) Quantification of microvessel capillaries in the Matrigel plugs. The data represent the mean ± SEM; n = 5-10 from 3-5 independent experiments; *P < .05 and **P < .01 compared with control or as indicated. (I-K) Thin cryosections (5 μm) prepared from Matrigel plugs were stained with anti–human von Willebrand Factor (red) or anti–mouse CD31(green) to distinguish human and mouse ECs. Highly autofluorescent red blood cells inside neovessels are yellow in color. Note that close association between human and murine ECs are indicated by white arrows. Green arrows indicate HUVECs that failed to form blood vessels; yellow arrow indicates neovessel made of HUVECs. Original magnification, ×200. Epifluorescence images were captured using a Zeiss Axioplan 2 inverted microscope under 20× objectives, using an AxioCam H digital camera. Digital images were saved as TIFF documents using Adobe Photoshp CS. Multiple images were assembled using QuarkXpress 8.0 software and labeled, and final images were saved as EPS documents.
NANOG knockdown decreases angiogenesis in Matrigel plug assays. (A) Timeline of the Matrigel plug assay. (B-D) HUVECs were incubated with the indicated shRNAs (retrovirus) for 10 hours (in presence of serum and growth factors), after which time the cells (105) were mixed with growth facto–reduced Matrigel (250 μL) plus WNT3A (50 ng/mL) and injected subcutaneously into athymic nude mice (n = 5). After 7 days, the Matrigel plugs were collected, embedded in paraffin, sectioned (4-5 μm), and stained with hematoxylin and eosin. Pictures of Matrigel plugs were taken using a Canon Powershot A640 digital camera controlled by Zoom Browser Ex software 5.7. Digital images were then saved as EPS documents using Adobe Photoshop CS. Multiple images were assembled using QuarkXpress 8.0 software and labeled, and final images were saved as EPS documents. (E-G) Note the prominent microvessels in the control shRNA (noninterfering shRNA retrovirus) plugs and the reduced number of capillaries in the NANOG knockdown plugs. FLK1 cDNA expression partially compensated for the loss of NANOG. Scale bar, 40 μm. (H) Quantification of microvessel capillaries in the Matrigel plugs. The data represent the mean ± SEM; n = 5-10 from 3-5 independent experiments; *P < .05 and **P < .01 compared with control or as indicated. (I-K) Thin cryosections (5 μm) prepared from Matrigel plugs were stained with anti–human von Willebrand Factor (red) or anti–mouse CD31(green) to distinguish human and mouse ECs. Highly autofluorescent red blood cells inside neovessels are yellow in color. Note that close association between human and murine ECs are indicated by white arrows. Green arrows indicate HUVECs that failed to form blood vessels; yellow arrow indicates neovessel made of HUVECs. Original magnification, ×200. Epifluorescence images were captured using a Zeiss Axioplan 2 inverted microscope under 20× objectives, using an AxioCam H digital camera. Digital images were saved as TIFF documents using Adobe Photoshp CS. Multiple images were assembled using QuarkXpress 8.0 software and labeled, and final images were saved as EPS documents.
Discussion
NANOG is considered to be the “gateway to the pluripotent ground state.”35 The ability of NANOG to promote stem cell self-renewal and proliferation and to generate induced pluripotent stem cells has been well established.17,18,20,35-37 In this study, we have identified a novel role of NANOG in regulating FLK1 expression and angiogenic activity of ECs.
Here, we show that NANOG is expressed in low levels in ECs and in a subset of tumor cell lines. Although the expression of NANOG is thought to be reduced in differentiated cells and tissues, our data show that NANOG is in fact present in mature ECs. We confirmed the expression of NANOG in ECs not only by WB analysis but also by RT-PCR and quantitative RT-PCR. We also determined the NANOG expression in hESCs vis a vis HUVECs, with and without WNT stimulation. Importantly, NANOG was not only expressed in cultured ECs but also in sprouting ECs and hematopoietic cells in the E14.5-day-old embryo. We detected NANOG at appreciable levels in randomly growing ECs (HUVECs, LMVECs, and HdMVECs), Jurkat (human T-cell leukemia), and MCF7 (human breast carcinoma) cells but not in A431 (epidermoid carcinoma), HeLa (cervical carcinoma), SW480 (colon carcinoma), or MRC-5 lung fibroblast cells. The reason for differential NANOG expression is not clear, but one can surmise that NANOG present in some tumor cells provides a proliferative advantage to these cells or it may reflect the presence of NANOG+ cancer stem cells in these lines. On the basis of anti-Nanog immunostaining of an E14.5-day-old embryo, our data show that Nanog is expressed in sprouting ECs. Thus, Nanog is not only expressed in cultured ECs but also detectable in sprouting ECs in vivo.
We next evaluated the NANOG-mediated transcriptional regulation of FLK1, a key determinant of EC proliferation, differentiation, migration, and survival.38 In growth factor–starved HUVECs, NANOG was not detectable by WB analysis, whereas baseline NANOG expression levels seen in HUVECs cultured in the presence of growth factors and serum (2%). Thus, the HUVECs were not starved of growth factors in subsequent experiments. Despite the presence of serum, NANOG expression was significantly increased in response to LiCl and WNT3A stimulation. Wnt ligation induces the stabilization of β-catenin,3-11 which was found to be up-regulated along with NANOG and FLK1 in HUVECs. Given that β-catenin is downstream of WNT3A signaling,18-21 it is possible that WNT3A can induce the expression of KLF4, which then induces NANOG expression,25-28,34,39 whereas NANOG, in turn, can induce the expression of FLK1 in HUVECs. Our data also showed the ability of serum factors to regulate NANOG expression in these cells; currently, however, the mechanisms underlying this effect are not clear. In other words, NANOG is detectable in growing ECs, without WNT3A addition. Although we found that NANOG knockdown decreases the expression of FLK1 and reduces cell proliferation, we cannot rule out the possibility that NANOG also regulates the G1-to-S phase transition of the EC cycle.40
Given that the presence of flk1 is required for the development of ECs from primitive hemangioblasts,24 we examined whether NANOG interacts with the FLK1 promoter/enhancer region in ECs. Both the human and mouse FLK1 promoters contain binding sites for transcription factors such as activator protein-2, nuclear factor κ-light-chain enhancer of activated B cells, specific protein-1, and KLF2.41,42 To establish a relationship between NANOG and the human FLK1 promoter, we examined the −1.5-kb promoter sequence and identified 8 ATTA sites as potential NANOG binding elements.20,35 The results of ChIP and EMSA experiments showed that NANOG binds to ≥ 4 of these ATTA sites. We observed a strong correlation between increased NANOG expression and NANOG binding to the NANOG promoter/enhancer region. We have also observed the binding of NANOG to the CYCLIN-D1 promoter by EMSA (data not shown). Thus, our data suggest that NANOG induces EC proliferation and angiogenesis by binding to and activating the FLK1 and CYCLIN-D1 promoters in response to WNT3A stimulation. Despite a > 3-fold increase in FLK1 protein expression after FLK1-cDNA transfection into the NANOG knockdown ECs; however, the effect of NANOG depletion was not completely reversed in these cells. FLK1 cDNA transfection into NANOG-depleted ECs restored basal expression of CYCLIN-D1; however, transfection of CYCLIN-D1 cDNA into NANOG-depleted ECs remained inconclusive (data not shown). Thus, these findings suggest that NANOG target genes in ECs directly contribute to the angiogenic activity of ECs. The transcription factor KLF2 has been shown to bind the FLK1 promoter downstream of exon 1, thereby down-regulating FLK1 expression and playing an atheroprotective role.42 Thus, it is probably that the regulation of the human FLK1 promoter is complex, takes place at several levels, and is coordinated by multiple transcription factors.43,44 The identification and characterization of regulatory mechanisms in addition to NANOG that are responsible for FLK1 expression could show clues about the specification and differentiation of ECs during development and pathogenesis. Surprisingly, NANOG depletion not only decreased FLK1 expression but also reduced cell proliferation and angiogenesis. Accordingly, expression of human FLK1 cDNA in the NANOG-depleted ECs partially reversed the effect of NANOG depletion in an in vivo Matrigel plug assay of angiogenesis, suggesting that NANOG probably has other targets in ECs. Interestingly, after NANOG depletion, the proangiogenic growth factor, platelet derived growth factor BB was down-regulated, whereas antiangiogenic factor tissue inhibitor of metalloproteinase 1 was up-regulated significantly (*P < .5) (supplemental Figure 6). Thus, NANOG may not only be acting through FLK1 and CYCLIN-D1, but it may also be required for optimal production of proangiogenic factors and thereby optimal angiogenic activities of ECs.
It remains unclear why ECs express NANOG. There is no single transcription factor that specifically regulates the angiogenic activity of ECs,43,44 and it is probable that the gene dosage and expression kinetics of several key transcription factors orchestrate angiogenesis in a temporospatial manner. Given that the Wnt and transforming growth factor-β1 signaling pathways induce de-differentiation of ECs into mesenchymal-like cells,45,46 NANOG expression in these cells may serve to induce an endothelial stem transition–like state. Our findings may therefore have a number of implications for the biology of cells such as ECs that express high levels of NANOG. For example, NANOG expression may signal the induction of a “stem-like” state in a subset of cells. If this is true, then transient induction of stemness in a subset of ECs may generate stem cells. Particularly, the use of chemical compounds or genetic means to transiently induce NANOG expression in a population of normal ECs may provide a means of generating large numbers of normal progenitor endothelial or stem-like cells for transplantation and regenerative medicine.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sakina Petiwala and Kevin Shyu for their excellent technical assistance.
These studies were supported by the National Institutes of Health (NIH; R01HL079356; HL079356-03S1), American Heart Association grants, and by the University of Illinois at Chicago Center for Clinical and Translational Science Award Number UL1RR029879 from the National Center for Research Resources to K.K.W. C.E.C. was supported by a T32HL072742 NIH training grant and E.E.K. was supported by a T32GM070388 NIH training grant.
National Institutes of Health
Authorship
Contribution: E.E.K. designed the oligonucleotides; purified and biotinylated the probes; characterized the NANOG binding sites with the use of EMSA and reporter assays; performed the knockdown, Western blot, and expression analyses and Matrigel plug assays; collected data and prepared the manuscript. C.E.C. performed the Western blot and RT-PCR experiments. I.C. generated shRNA, performed knockdown experiments and Western blot, collected conditioned media, and analyzed ELISA data. A.B.M. prepared the manuscript. K.K.W. conceived the hypothesis, designed experiments, performed the Matrigel plug assays, analyzed and interpreted the data, and prepared and communicated the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kishore K. Wary, Department of Pharmacology, University of Illinois, 835 S Wolcott Ave, Rm E403, Mail code 868, Chicago, IL 60612; e-mail: kkwary@uic.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal