Abstract
We generated a transgenic mouse line that expresses the Cre recombinase under the control of the Ncr1 (p46) promoter. Cre-mediated recombination was tightly restricted to natural killer (NK) cells, as revealed by crossing Ncr1-iCreTg mice to the eGFP-LSLTg reporter strain. Ncr1-iCreTg mice were further used to study NK cell–specific functions of Stat5 (signal transducers and activators of transcription 5) by generating Stat5f/fNcr1-iCreTg animals. Stat5f/fNcr1-iCreTg mice were largely devoid of NK cells in peripheral lymphoid organs. In the bone marrow, NK-cell maturation was abrogated at the NK cell–precursor stage. Moreover, we found that in vitro deletion of Stat5 in interleukin 2–expanded NK cells was incompatible with NK-cell viability. In vivo assays confirmed the complete abrogation of NK cell–mediated tumor control against B16F10-melanoma cells. In contrast, T cell–mediated tumor surveillance against MC38-adenocarcinoma cells was undisturbed. In summary, the results of our study show that STAT5 has a cell-intrinsic role in NK-cell development and that Ncr1-iCreTg mice are a powerful novel tool with which to study NK-cell development, biology, and function.
Introduction
Natural killer (NK) cells are members of the innate immune system and represent a third lineage of lymphoid cells distinct from T and B lymphocytes. NK cells were initially discovered through their ability to spontaneously lyse tumor cells.1 The importance of NK cells in tumor surveillance has been shown in vitro and in vivo in different mouse models.2,3 Moreover, NK cells recognize and eliminate cells infected by certain viruses or parasites,4,5 and produce and secrete cytokines such as interferonγ (IFNγ) and tumor necrosis factor (TNF), which stimulate the adaptive and innate immune responses.6 Therefore, NK cells exert an important function in orchestrating the interplay of innate and adaptive immunity.
In adult mice, NK-cell differentiation takes place mainly in the bone marrow.7 The earliest NK-cell precursors (NKPs) are characterized by the expression of the interleukin 2 (IL2) and IL15 receptor common β subunit, also known as CD122, and the absence of NK-lineage markers such as the NK1.1, DX5, and Ly49 receptors.8 This cell type gives rise to immature NK (iNK) cells, which are positive for NK1.1, negative for DX5, and display reduced expression of certain Ly49 receptors. Further differentiation comprises mature NK (mNK) cells expressing NK1.1, DX5, and Ly49 receptors. Mature NK cells may leave the bone marrow and migrate to secondary lymphoid organs, lung, liver, and gut. Recently, IL22-producing lymphoid cells in the intestinal lamina propria have been characterized that are positive for NCRI (natural cytotoxicity receptor I), NKG2D, and NK1.1 and express the orphan transcription factor RORyt.9 However, NKPs and iNKs are not uniquely restricted to the bone marrow because they have been found at other sites such as the spleen10 and lymph nodes.11 It has therefore been suggested that multiple sites may support NK-cell differentiation or, alternatively, that NKPs and iNKs from the bone marrow have access to the circulation. As NK cells mature, they sequentially acquire their characteristic NK cell–receptor repertoire.12 NCRI, also known as NKp46, becomes expressed during the early iNK-cell stage and remains constitutively expressed.13,14 The ligand(s) of this receptor has only been partially characterized.15,16 NCRI is involved in the control of influenza infection by recognizing the viral hemagglutinin protein,13 and most recently has been identified as factor modulating disease progression in type 1 diabetes.17 The developmental pathways generating NK-cell diversity, including the transcriptional machinery behind them, are not well understood and remain elusive.
The differentiation and homeostasis of lymphocytes are regulated by cytokines such as the interleukins IL2, IL4, IL7, IL15, and IL21. All of these cytokines require the common γ chain (γc) and activate major signaling pathways, such as that involving the Janus family of tyrosine kinases (JAKs) and signal transducers and activators of transcription (STATs), thereby contributing to the biological effects of lymphoid cells.18 The JAKs are stably associated with the cytokine receptor and induce the activation of STATs upon receptor stimulation, with STAT5 being predominantly activated by IL2, IL7, and IL15.19 STAT5 consists of 2 highly homologous isoforms, STAT5A and STAT5B, which are encoded by separate genes. In the lymphoid system, STAT5A and STAT5B fulfill largely redundant roles, although STAT5B has been implicated in NK-cell development.20 In Stat5b−/− mice, NK-cell numbers were found to be reduced to 50%, and whole splenocyte cultures showed a reduced cytolytic capacity in response to IL2 and IL15 that was attributed to the NK-cell compartment.21
The generation of mice deficient for both Stat5a and Stat5b (Stat5a/b) verified their important role in lymphoid development and homeostasis. The first Stat5a/b-knockout mouse expressed N-terminally truncated proteins at various expression levels, depending on the tissue type, and led to a viable phenotype (now referred to as Stat5ΔN/ΔN mice). The residual STAT5ΔN proteins bind DNA and activate the transcription of some but not all target genes.20 Furthermore, STAT5ΔN proteins are constitutively active even in the absence of cytokines. Therefore, the interpretation of phenotypes obtained with Stat5ΔN/ΔN mice is complex and requires revalidation in a model completely devoid of STAT5 proteins. This can be most obviously seen by the fact that mice lacking complete Stat5a/b die perinatally, whereas Stat5ΔN/ΔN animals are viable.22 Stat5a/bf/f mice (henceforth Stat5f/f mice) crossed to B and T cell–specific Cre lines revealed multiple and complex functions of STAT5A/B (henceforth STAT5). In B cells, STAT5 mediates survival downstream of IL7 and is involved in immunoglobulin rearrangement and pre-B–cell expansion.23 Within the T-lymphoid lineage, STAT5 is mainly required for the expansion of CD8+ T cells and T-cell receptor γδ (TCRγδ) lymphocytes.24 The key role of STAT5 in lymphoid cells is also underlined by the fact that STAT5 is constitutively active in many lymphoid malignancies, which may even critically depend on its presence.25
In this study, we describe the successful generation of a mouse model that allows for the first time conditional mutagenesis specifically in NK cells. Our work reveals a critical role for the transcription factor STAT5 in NK-cell development and survival. In Stat5f/fNcr1-iCreTg mice, NK cells are nearly completely absent, causing the virtual abrogation of NK cell–mediated tumor surveillance without affecting T cell–controlled immune surveillance.
Methods
Generation of Ncr1-iCreTg transgenic mice
Codon-improved Cre (iCre) recombinase was inserted into a bacterial artificial chromosome (BAC; RP23-267N11; purchased from Children's Hospital Oakland Research Institute, Oakland, CA) harboring the Ncr1 gene via homologous recombination in Escherichia coli.26 Briefly, a cassette containing iCre recombinase, an artificial intron, a bovine growth hormone polyadenylation signal, and an ampicillin-resistance gene flanked by FRT (Flp recombinase target) sites was recombined into the first exon of the Ncr1 gene. Correctly recombined BACs were transiently electroporated with a plasmid expressing the Flp recombinase to delete the ampicillin gene. The BACs were verified by Southern blot and sequencing. BAC DNA was digested with NotI, purified using a Sepharose CL4b column, and injected into the pro-nuclei of C57BL/6N oocytes. Three of 4 Tg(Ncr1-iCre)264-267 founders expressed the transgene, and Tg(Ncr1-iCre)265 was selected for detailed analysis. Genotyping of Tg(Ncr1-iCre)265 mice (henceforth Ncr1-iCreTg) was performed using the following primers: 5′GACCATGATGCTGGGTTTGGCCCAGATG and 5′ATGCGGTGGGCTCTATGGCTTCTG yielding a 500 bp polymerase chain reaction (PCR) product.
Mice
Antibodies and flow cytometric analysis
The following antibodies were purchased from BD Biosciences: NK-1.1 (PK136), CD49b (HMα2), CD3e (145-2C11), CD3 (17A2), CD4 (RM4-5), CD8a (53-6.7), CD45R/B220, CD19, γδ TCR (GL3), TCRβ (H57-597), CD122 (TM-beta 1), Ly6G/Ly6C (RB6-8CM), CD3e (145-2C11), TER119, CD45R/B220(RA3-6B2), CD27 (LG3A10), CD11b (M1/70), CD44 (IM7), Ly49A (A1), NKp46 (29A1.4), Ly49 D (4E5), KLRG1 (2F1), and CD25 (Pc61). NKp46 (29A1.4), Ly49H (3D10), and NKG2D (CX5) were obtained from eBioscience. PBS57-loaded and -unloaded CD1d tetramers were generously donated by Wilfried Ellmeier (Medical University, Vienna, Austria). For flow cytometry, single-cell suspensions were prepared and splenocytes were depleted of red blood cells. Purified rat anti–mouse CD16/CD32 (2.4G2; BD Pharmingen) was added to avoid nonspecific binding. For intracellular staining, cells were fixed with 2% paraformaldehyde at room temperature for 10 minutes, and incubated with ice-cold methanol for 20 minutes at −20°C. Fc receptors were blocked, and cells were incubated with STAT5 antibody (C17; Santa Cruz Biotechnology) at 4°C overnight under agitation and counterstained with phycoerythrin-conjugated goat anti–rabbit immunoglobulin G (Santa Cruz Biotechnology). For in vivo bromodeoxyuridine (BrdU)–incorporation assays, mice were intraperitoneally injected with 1 mg of BrdU (in 100 μL). After 12 hours, splenocytes were isolated, stained, fixed, permeabilized, and treated with DNase. Analysis of BrdU incorporation was performed using the BrdU Flow Kit (BD Pharmingen). Stained samples were analyzed using FACSCantoII and FACSDiva software Version 6.1.2 (BD Biosciences).
NK-cell purification, expansion, and function
NK cells were purified and expanded as described previously.28 Briefly, single-cell suspensions were prepared from ≥ 4 spleens/genotype. For NK-cell purification, cell suspensions were incubated with anti-DX5–coated MACS beads (Miltenyi Biotec) and purified on a MACS separator (Miltenyi Biotec). NK cells were expanded for 6-12 days in RPMI 1640 medium containing 10% fetal calf serum (FCS), β-2ME, l-glutamine, and penicillin/streptomycin supplemented with rhIL2 (henceforth IL2; 5000 U/mL). The purity as assessed by flow cytometry was > 90%-95%. For cell sorting, splenocytes were depleted of red blood cells, incubated with anti–CD3 and anti–DX5, and sorted into a CD3−DX5+ population. For in vitro proliferation assays, purified NK cells at day 6 were seeded in flat-bottom, 96-well plates (1 × 105 cells/well) under IL2 (5000 U/mL). Proliferation was measured by 3[H]-thymidine incorporation. NK-cell cytotoxicity was analyzed by flow cytometry, as described previously.29 Briefly, 1 × 104 target cells/well were incubated with 5μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) for 6 minutes at 4°C in the dark. Expanded NK cells at day 10 were coincubated with CFSE-labeled YAC-1, RMA, and RMA-Rae1γ targets at the indicated E:T ratios for 4 hours at 37°C and placed on ice. Propidium iodide (Sigma-Aldrich) was added to each well immediately before flow cytometry. The percentage of specific lysis was determined as described previously.29 To determine the effects of Stat5 deletion in primary NK cells, Stat5f/f NK cells were purified, expanded for 3 days under IL2, and infected with adenovirus expressing either green fluorescent protein (GFP) or Cre-GFP (kindly provided by Wolfgang Mikulits, Medical University, Vienna, Austria) and 7 μg/mL of polybrene. GFP expression of CD3−DX5+ cells was tracked via flow cytometry. The day of the highest GFP expression was defined as day 0.
T-cell stimulation
MACS-sorted (Miltenyi Biotec) T cells (5 × 105 cells/well) were stimulated with 1 μg/mL of plate-bound anti–CD3ϵ (145-2C11; BD Biosciences) and 2 μg/mL of anti–CD28 (37.51; BD Biosciences) on 48-well plates in the presence of 100 U/mL of IL2 for 48 hours. T cells were harvested after 5 days and analyzed by flow cytometry.
Cell lines and tumor models
B16F10 and MC38 cell lines were maintained in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated FCS, 100 U/mL of penicillin-streptomycin, 2mM l-glutamine, and 5μM β-mercaptoethanol. YAC-1, RMA, and RMA-Rae1γ cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 100 U/mL of penicillin-streptomycin, 2mM l-glutamine, and 5μM β-mercaptoethanol. Mice were injected intravenously with 1 × 106 B16F10 and subcutaneously with MC38, and monitored daily for disease onset. Tissues were isolated, weighed, fixed in 3.7% formaldehyde, and analyzed. For NK1.1+ and CD8+ T-cell depletion, 100 μg of anti–NK1.1 antibody and anti–CD8 antibody was injected twice a week starting 2 days before beginning the experiment. Anti–NK1.1 antibody and anti–CD8 antibody were purified from the PK136-cell and 53-6.72-cell supernatants, respectively. The deletion was confirmed by flow cytometry of splenocytes.
Real-time PCR
Total RNA was isolated using TRI reagent (Sigma-Aldrich) according to the manufacturer's instructions. One microgram of total RNA was used for cDNA synthesis using the GeneAmp RNA PCR kit (Roche) and for the real-time PCR reaction performed on an Eppendorf RealPlex cycler using Taq DNA polymerase (5Prime) and SYBR Green. All experiments were performed in triplicate. The following primers were used: Stat5a Forward: CAGATCAAGCAAGTGGTCCC3′, Stat5a Reverse: TCGAGACTGTCCATGGGCC, Stat5b Forward: GGCAGGGTCAGTAACGGAAG, Stat5b Reverse: GGCTCTGCAAAGGCGTTGTC. Samples were normalized to GAPDH expression; Gapdh Forward: TCTCCTCTGACTTCAACAGCG, Gapdh Reverse: ACCACCCTGTTGCTGTAGCC
Statistics
Statistical analysis was performed using Student t test except for the results shown in Figure 5B and C, for which a Mann Whitney U test was used (**P = .0014). The P values were defined as follows: *P < .05; **P < .01; and ***P < .001. Data are expressed as mean ± SEM and were analyzed with Prism software Version 5.03 (GraphPad).
Results
Generation and characterization of Ncr1-iCreTg mice
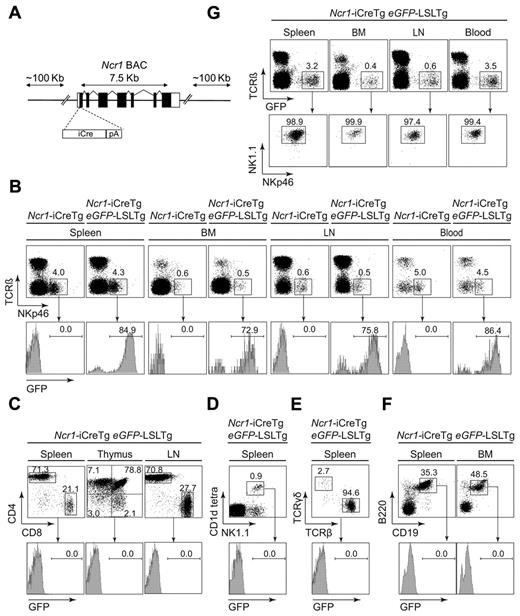
To restrict Cre recombinase expression to NK cells, we generated transgenic mice expressing Cre recombinase under the control of the Ncr1 promoter (schematically illustrated in Figure 1A). We used a BAC clone containing the entire Ncr1 gene, along with abundant upstream and downstream flanking DNA. By homologous recombination in bacteria, the coding part of the first exon of Ncr1 was replaced by the iCre expression cassette. To test the functionality and lineage specificity, Ncr1-iCreTg–transgenic mice were crossed to the eGFPLSL-reporter mouse line that expresses enhanced GFP (eGFP) upon Cre-mediated excision of a loxP-flanked stop cassette (see also supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).27 As depicted in Figure 1B, eGFP expression in TCRβ−NKp46+ NK cells varied between 70% and 90% in individual Ncr1-iCreTg eGFP-LSLTg double-transgenic mice. On average, we observed 81.0% ± 2.42% eGFP+ NK cells in the spleen, 71.9% ± 1.56% in the bone marrow, 74.0% ± 2.41% in the lymph nodes, and 80.0% ± 2.75% in the blood (summarized in supplemental Figure 2). No eGFP expression was detected in T-lymphoid cells, as analyzed by CD3, CD4, and CD8 staining (Figure 1C). Despite the general agreement that NCR1 is constitutively and selectively expressed in NK cells,30 it is also known to be expressed in peripheral NK-like TCRγδ cells.31 Therefore, to unequivocally define eGFP expression in these cells, we stained splenocytes from Ncr1-iCreTg eGFP-LSLTg mice for NK1.1 and CD1d tetramer and for TCRαβ and TCRγδ. As depicted in Figure 1D and E, we failed to observe any eGFP expression in NKT and TCRγδ cells. Similarly, we failed to detect eGFP+ B-lymphoid cells (Figure 1F). In addition, we confirmed the exclusive Ncr1-dependent Cre recombination by staining of splenocytes with TCRβ, NK1.1, and NKp46. As expected, eGFP+ cells were restricted to the NK-cell compartment (TCRβ−NK1.1+NKp46+; Figure 1G). We therefore concluded that Cre recombination in Ncr1-iCreTg mice is restricted to NK cells.
Generation and characterization of Ncr1-iCreTg mice. (A) Schematic illustration of the modified Ncr1 BAC. iCre cDNA was inserted by homologous recombination into the exon containing the translation initiation codon of a BAC harboring the mouse Ncr1 gene. (B) Efficiency of Cre-mediated eGFP expression verified via flow cytometry of Ncr1-iCreTg eGFP-LSLTg double-transgenic mice and littermate controls. Excision of a stop cassette flanked by loxP sites leads to eGFP expression that can be analyzed by flow cytometry. Numbers adjacent to outlined areas indicate the percentage of NK cells (TCRβ−NKp46+) of various lymphoid organs. Histograms show the percentage of eGFP expression of gated NK cells. (C-F) Flow cytometry of Ncr1-iCreTg eGFP-LSLTg double-transgenic mice and their littermate controls showing the absence of eGFP expression in hematopoietic cell lineages other than NK cells. Dot plots indicate the percentages of (C) gated CD3+ CD4+CD8+ cells, (D) gated CD3− NK1.1+CD1d tetra+ cells, (E) gated CD3+ TCRγδ+ TCRβ+ cells, and (F) B220+CD19+ cells. Histograms show the percentage of eGFP expression. (G) Almost all NK cells express eGFP. Approximately 99% of gated TCRβ −eGFP+ cells are NK cells (NK1.1+NKp46+; n ≥ 4 per genotype). Data are representative of at least 3 independent experiments. BM, bone marrow; LN, lymph nodes.
Generation and characterization of Ncr1-iCreTg mice. (A) Schematic illustration of the modified Ncr1 BAC. iCre cDNA was inserted by homologous recombination into the exon containing the translation initiation codon of a BAC harboring the mouse Ncr1 gene. (B) Efficiency of Cre-mediated eGFP expression verified via flow cytometry of Ncr1-iCreTg eGFP-LSLTg double-transgenic mice and littermate controls. Excision of a stop cassette flanked by loxP sites leads to eGFP expression that can be analyzed by flow cytometry. Numbers adjacent to outlined areas indicate the percentage of NK cells (TCRβ−NKp46+) of various lymphoid organs. Histograms show the percentage of eGFP expression of gated NK cells. (C-F) Flow cytometry of Ncr1-iCreTg eGFP-LSLTg double-transgenic mice and their littermate controls showing the absence of eGFP expression in hematopoietic cell lineages other than NK cells. Dot plots indicate the percentages of (C) gated CD3+ CD4+CD8+ cells, (D) gated CD3− NK1.1+CD1d tetra+ cells, (E) gated CD3+ TCRγδ+ TCRβ+ cells, and (F) B220+CD19+ cells. Histograms show the percentage of eGFP expression. (G) Almost all NK cells express eGFP. Approximately 99% of gated TCRβ −eGFP+ cells are NK cells (NK1.1+NKp46+; n ≥ 4 per genotype). Data are representative of at least 3 independent experiments. BM, bone marrow; LN, lymph nodes.
Cre expression does not alter NK-cell development and function
Although Cre recombinase has been intensively used to induce genomic recombination, Cre expression has been reported to be toxic for some eukaryotic cells.32,33 This may be related to chromosomal rearrangements caused by recombination between cryptic “pseudo-loxP” sites naturally occurring within the genome. Alternatively, integration of the transgene may disrupt the genes important for organ and/or cell development. Therefore, we next studied the effects of Cre expression per se on NK-cell development and function. Ncr1-iCreTg mice were born at the expected Mendelian ratio without any visible alterations in organ morphology or overt pathology (data not shown). NK cells develop mainly in the bone marrow. Basically, 3 major developmental stages can be distinguished by their NK1.1 and DX5 expression.7 NKPs at the first developmental stage are negative for NK1.1 and DX5, iNKs express NK1.1 but not DX5, and mNKs are positive for both NK1.1 and DX5. At the iNK cell stage, Ncr1 is expressed and remains expressed throughout all stages14 (schematically illustrated in Figure 2A). No changes in NKPs (Lin−CD122+NK1.1−DX5−) in the bone marrow of Ncr1-iCreTg mice were detected compared with wild-type (WT) littermate controls (Figure 2B; absolute cell numbers are shown in supplemental Figure 3A). Moreover, peripheral mature NK cells were present at equal levels in the spleen, lymph nodes, and blood, as depicted in Figure 2C (absolute cell numbers are provided in supplemental Figure 3B). No difference in the mean fluorescence intensity of NKp46 was detected (supplemental Figure 4). Mature NK cells in the spleen are further subdivided into functionally distinct subsets depending on CD27 and CD11b expression. A 4-stage process from CD11blowCD27low to CD11blowCD27high to CD11bhighCD27high to CD11bhighCD27low is thought to reflect the developmental program associated with a progressive acquisition of NK-cell effector functions.34 Again, analysis of the surface markers CD27 and CD11b revealed an unaltered NK-cell maturation in Ncr1-iCreTg mice (Figure 2D). Similarly, in vivo NK-cell proliferation in the spleen using BrdU incorporation and in vitro proliferation of IL2-cultured NK cells was unaffected (Figure 2E-F). Finally, given that lysis of target cells is a major NK-cell function, we performed in vitro cytotoxicity assays using MACS-purified, IL2-expanded splenic NK cells derived from Ncr1-iCreTg mice and their littermate controls. Figure 2G summarizes our efforts. YAC-1 cells that express low levels of major histocompatibility complex I (MHC I) were used as target cells, as well as RMA-Rae1γ cells expressing the NKG2D ligand Rae-1.35 RMA cells served as a negative control because they are not efficiently lysed by syngeneic NK cells. In summary, all results were similar irrespective of the target cells used. No differences in the killing activity of Ncr1-iCreTg and their littermate controls were detectable. Overall, these experiments suggest that Cre expression in NK cells impairs neither NK-cell development nor NK-cell function.
Development and function is not altered in Cre-expressing NK cells. Neither the development nor the proliferation or cytotoxicity of NK cells was influenced by Cre recombinase. (A) Simplified scheme of NCRI expression in NK-cell development. (B-E) Flow cytometry of Ncr1-iCreTg mice and littermate controls. Dot plots indicate the percentage of (B) gated Lin−CD122+ DX5−NK1.1− NKPs in the BM, (C) gated CD3− NK cells (NK1.1+DX5+) in the periphery, and (D) expression of maturation markers of splenic NK cells gated as TCRβ−NK1.1+ stained with CD27 and CD11b antibodies (n ≥ 4 per genotype). Data are representative of at least 3 independent experiments. (E) In vivo proliferation of splenic NK cells. Mice were injected intraperitoneally with BrdU and after 12 hours, the incorporation of BrdU in splenic NK cells was analyzed. Numbers adjacent to outlined areas in the dot plot indicate the percentage of CD3−NK1.1+ cells. Histograms show the percentage of BrdU-positive cells (n ≥ 5 per genotype). Data are representative of 2 independent experiments. (F) In vitro proliferation of IL2-expanded NK cells purified from the spleens of mice of the indicated genotypes. At day 6, NK cells were seeded in triplicate in 96-well plates. After 12 hours, proliferation was measured by standard 3[H]-thymidine incorporation. Four mice per genotype were pooled. Data are representative of 2 independent experiments. (G) Cytotoxicity of IL2-expanded splenic NK cells purified from indicated genotypes. At day 10, NK cells were coincubated in triplicate with CFSE-labeled YAC-1, RMA-Rae1γ, and RMA targets at the indicated E:T ratios. Five mice per genotype were pooled. Data are representative of 2 independent experiments.
Development and function is not altered in Cre-expressing NK cells. Neither the development nor the proliferation or cytotoxicity of NK cells was influenced by Cre recombinase. (A) Simplified scheme of NCRI expression in NK-cell development. (B-E) Flow cytometry of Ncr1-iCreTg mice and littermate controls. Dot plots indicate the percentage of (B) gated Lin−CD122+ DX5−NK1.1− NKPs in the BM, (C) gated CD3− NK cells (NK1.1+DX5+) in the periphery, and (D) expression of maturation markers of splenic NK cells gated as TCRβ−NK1.1+ stained with CD27 and CD11b antibodies (n ≥ 4 per genotype). Data are representative of at least 3 independent experiments. (E) In vivo proliferation of splenic NK cells. Mice were injected intraperitoneally with BrdU and after 12 hours, the incorporation of BrdU in splenic NK cells was analyzed. Numbers adjacent to outlined areas in the dot plot indicate the percentage of CD3−NK1.1+ cells. Histograms show the percentage of BrdU-positive cells (n ≥ 5 per genotype). Data are representative of 2 independent experiments. (F) In vitro proliferation of IL2-expanded NK cells purified from the spleens of mice of the indicated genotypes. At day 6, NK cells were seeded in triplicate in 96-well plates. After 12 hours, proliferation was measured by standard 3[H]-thymidine incorporation. Four mice per genotype were pooled. Data are representative of 2 independent experiments. (G) Cytotoxicity of IL2-expanded splenic NK cells purified from indicated genotypes. At day 10, NK cells were coincubated in triplicate with CFSE-labeled YAC-1, RMA-Rae1γ, and RMA targets at the indicated E:T ratios. Five mice per genotype were pooled. Data are representative of 2 independent experiments.
NK cells are severely reduced in Stat5f/fNcr1-iCreTg mice
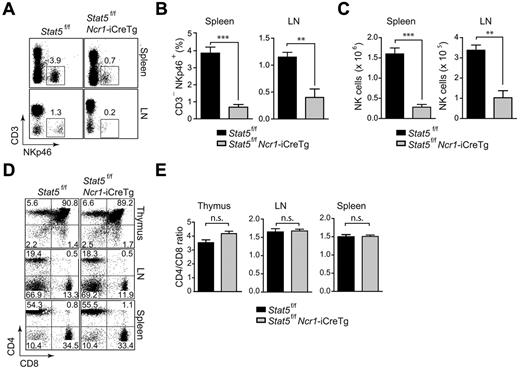
The transcription factor STAT5 is an important regulator of B- and T-lymphoid–cell development and function.23,24 To investigate the role of STAT5 in NK cells, we crossed Stat5f/f mice to Ncr1-iCreTg animals. Stat5f/fNcr1-iCreTg mice were born at the expected Mendelian ratio (data not shown). Hematopoietic organs displayed no gross abnormalities; weight and cellularity of spleens and cellularity of the thymi and bone marrow were unaltered in Stat5f/fNcr1-iCreTg mice compared with Stat5f/f littermate controls (data not shown and supplemental Figure 5). Analysis of CD3−NKp46+ cells in the spleens and lymph nodes revealed a severe reduction of NK cells in Stat5f/fNcr1-iCreTg mice. The NK-cell population was almost entirely absent (Figure 3A-B). Figure 3C shows the total NK-cell number in the spleens and lymph nodes. In contrast, no changes in the T-cell compartment were observed. The numbers of CD4+ and CD8+ T lymphocytes and CD4/CD8 ratios were unaltered in thymi and secondary lymphoid organs (Figure 3D-E).
NK cells are severely reduced in Stat5f/fNcr1-iCreTg mice. Deletion of Stat5 results in severely diminished NK-cell numbers. (A,D) Flow cytometry of Stat5f/fNcr1-iCreTg mice and littermate controls. Dot plots indicate the percentage of (A) peripheral NK cells (CD3−NKp46+) and (D) CD4 and CD8 expression on cells from various lymphoid organs. Cells were gated on total thymocytes (top panel), CD3+ lymph node cells (middle panel), and CD3+ splenocytes (bottom panel). (B) Bar graphs show the percentage of peripheral NK cells (CD3−NKp46+). (C) Bar graphs show the total splenic (left) and lymph node (right) NK-cell numbers. (E) Bar graphs show the CD4-to-CD8 ratio of total thymocytes (left panel), CD3+ lymph node cells (middle panel), and CD3+ splenocytes (right panel). (A,D) Data are representative of 3 independent experiments (n ≥ 4 per genotype). (B,C,E) n ≥ 5 per genotype.
NK cells are severely reduced in Stat5f/fNcr1-iCreTg mice. Deletion of Stat5 results in severely diminished NK-cell numbers. (A,D) Flow cytometry of Stat5f/fNcr1-iCreTg mice and littermate controls. Dot plots indicate the percentage of (A) peripheral NK cells (CD3−NKp46+) and (D) CD4 and CD8 expression on cells from various lymphoid organs. Cells were gated on total thymocytes (top panel), CD3+ lymph node cells (middle panel), and CD3+ splenocytes (bottom panel). (B) Bar graphs show the percentage of peripheral NK cells (CD3−NKp46+). (C) Bar graphs show the total splenic (left) and lymph node (right) NK-cell numbers. (E) Bar graphs show the CD4-to-CD8 ratio of total thymocytes (left panel), CD3+ lymph node cells (middle panel), and CD3+ splenocytes (right panel). (A,D) Data are representative of 3 independent experiments (n ≥ 4 per genotype). (B,C,E) n ≥ 5 per genotype.
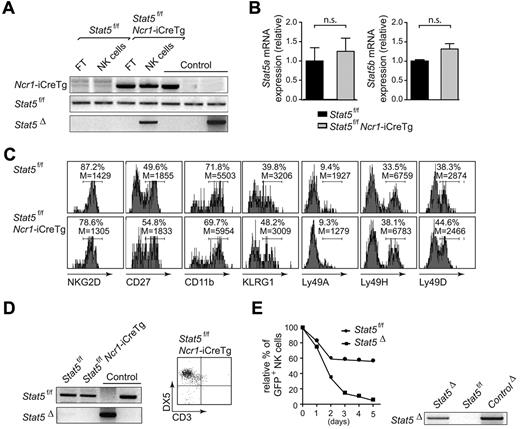
The few remaining purified NK cells from Stat5f/fNcr1-iCreTg spleens showed a PCR band indicative for the deletion of Stat5 that was present in neither the Stat5f/f fraction nor in the Stat5f/fNcr1-iCreTg NK cell–depleted flow-through. However, we also detected a PCR band for the floxed allele, indicating an incomplete deletion (Figure 4A). We concluded that the remaining NK cells represent a mixture of (1) NK cells that have not deleted the floxed allele (so-called “escapers”) and (2) NK cells that had just deleted the floxed allele, thus showing a deletion band. These “just deleters” would still possess STAT5 at the protein level, enabling their survival. The finding that there were comparable mRNA levels for STAT5a and STAT5b in the remaining NK cells compared with their controls suggests that the “escapers” were dominantly present in this mixture (Figure 4B). A comprehensive FACS analysis revealed an expression pattern of NK-cell markers comparable with that of the controls derived from Stat5f/f mice, making it unlikely that these remaining NK cells are immature or NK-like cells as described in IL15−/− and IL15Rα−/− mice,36,37 although we cannot entirely exclude this possibility (Figure 4C). After 10 days in culture under IL2, the few purified remaining NK cells were reanalyzed by FACS and PCR. At this time point, a deletion band could no longer be detected. All viable cells expressed Stat5 and showed a band indicative only for the floxed allele (Figure 4D left panel). The NK-cell nature of these cells was confirmed by their CD3−DX5+ surface expression (Figure 4D right panel). We reasoned that the absence of STAT5 is incompatible with NK-cell viability.
Loss of STAT5 is incompatible with NK-cell viability. (A) PCR genotyping of MACS-purified splenic NK cells of Stat5f/f and Stat5f/fNcr1-iCreTg mice. Four mice per genotype were pooled. (B) Real-time PCR analysis of Stat5a and Stat5b mRNA levels of FACS-sorted splenic CD3−DX5+ NK cells. Ten mice per genotype were pooled. (C) Histograms show percentage and mean fluorescence intensity of various NK-cell markers on CD3−NKP46+ splenocytes of the indicated genotypes. (D) MACS-purified splenic NK cells were cultured under IL2. After 10 days of culture, only those cells that expressed Stat5 expanded, as indicated by the lack of a Stat5 deletion band on PCR analysis (left panel). Flow cytometry confirmed the NK-cell nature of these cells (right panel). Dot plot indicates CD3−DX5+ cells. Four mice per genotype were pooled. (E) MACS-purified splenic NK cells from Stat5f/f mice were cultured under IL2 (4 mice per genotype were pooled) and infected with Ad/Cre-GFP (indicated as Stat5Δ) or mock-infected (indicated as Stat5f/f). Those cells that received the empty vector tolerated the expression of Ad/GFP, whereas those that had received Ad/Cre-GFP expressed the Cre recombinase and declined (left panel). PCR genotyping of the cells confirmed the deletion of Stat5 in Ad/Cre-GFP–infected NK cells (right panel). Data are representative of at least 2 independent experiments. FT, flow-through.
Loss of STAT5 is incompatible with NK-cell viability. (A) PCR genotyping of MACS-purified splenic NK cells of Stat5f/f and Stat5f/fNcr1-iCreTg mice. Four mice per genotype were pooled. (B) Real-time PCR analysis of Stat5a and Stat5b mRNA levels of FACS-sorted splenic CD3−DX5+ NK cells. Ten mice per genotype were pooled. (C) Histograms show percentage and mean fluorescence intensity of various NK-cell markers on CD3−NKP46+ splenocytes of the indicated genotypes. (D) MACS-purified splenic NK cells were cultured under IL2. After 10 days of culture, only those cells that expressed Stat5 expanded, as indicated by the lack of a Stat5 deletion band on PCR analysis (left panel). Flow cytometry confirmed the NK-cell nature of these cells (right panel). Dot plot indicates CD3−DX5+ cells. Four mice per genotype were pooled. (E) MACS-purified splenic NK cells from Stat5f/f mice were cultured under IL2 (4 mice per genotype were pooled) and infected with Ad/Cre-GFP (indicated as Stat5Δ) or mock-infected (indicated as Stat5f/f). Those cells that received the empty vector tolerated the expression of Ad/GFP, whereas those that had received Ad/Cre-GFP expressed the Cre recombinase and declined (left panel). PCR genotyping of the cells confirmed the deletion of Stat5 in Ad/Cre-GFP–infected NK cells (right panel). Data are representative of at least 2 independent experiments. FT, flow-through.
To substantiate these findings, we purified and cultivated NK cells derived from Stat5f/f animals. After 3 days of expansion under IL2, the cells were infected with an adenovirus expressing Cre recombinase (Ad/Cre-GFP) or mock-infected (Ad/GFP). Figure 4E summarizes our efforts. Whereas the NK cells tolerated the expression of Ad/GFP (indicated as Stat5f/f), NK cells that had received Ad/Cre-GFP had a disadvantage and declined (indicated as Stat5Δ; Figure 4E left panel). The deletion of Stat5 was confirmed by PCR analysis (Figure 4E right panel). In summary, these observations led us to conclude that STAT5 is required for NK-cell survival.
STAT5 is required for NK-cell development from the NKP to the iNK cell stage
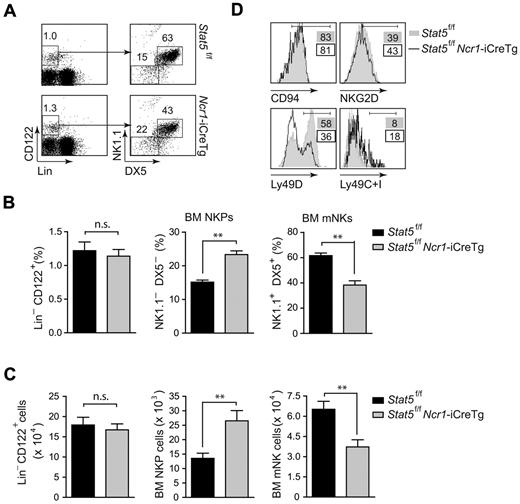
Our data so far indicated that the lack of STAT5 does not allow the survival of splenic NK cells and that STAT5 is indispensable for the viability of NK cells. We next investigated whether the lack of STAT5 would affect NK-cell differentiation in the bone marrow. As depicted in Figure 5A and B, flow cytometric analysis of Stat5f/f and Stat5f/fNcr1-iCreTg bone-marrow cells confirmed a significant decrease of mNK cells. In contrast, the Ncr1-iCreTg–dependent deletion of Stat5 was accompanied by an increase in NKP numbers. This increase in NKPs points to a developmental block occurring at the very first stage of NK-cell development, whereas no differences in Lin−CD122+ precursor cells were detected. Absolute cell numbers are shown in Figure 5C. Flow cytometric analysis of several NK-cell markers unveiled lower levels of the activatory receptor Ly49D, whereas the levels of the inhibitory receptors Ly49C and Ly49I were higher. In contrast, the levels of CD94 and NKG2D were comparable between the 2 genotypes. This finding reflects the immature nature of Stat5f/fNcr1-iCreTg–derived NK cells in the bone marrow (Figure 5D).
STAT5 is required for early NK-cell development in the bone marrow. (A) Flow cytometry of Stat5f/fNcr1-iCreTg mice and littermate controls. Representative dot plots indicate the percentage of gated Lin−CD122+ NKPs (DX5−NK1.1−) and gated Lin−CD122+ mNKs (DX5+NK1.1+) cells. (B) Bar graphs show the percentage of Lin−CD122+ cells, NKPs (DX5− NK1.1−, gated on Lin−CD122+), and mNKs (DX5+ NK1.1+, gated on Lin−CD122+) in the bone marrow (n = 8 STAT5f/f; n = 7 STAT5f/fNcr1-iCreTg, **P = .0014, Mann Whitney U test). (C) Bar graphs show absolute cell number of Lin−CD122+ cells, NKPs, and mNKs in the bone marrow (n = 8 STAT5f/f; n = 7 STAT5f/fNcr1-iCreTg, **P = .0014, Mann Whitney U test). (D) Histograms show the expression of indicated differentiation markers as percentages. (A-D) Data are representative of at least 3 independent experiments. (D) n ≥ 4 per genotype.
STAT5 is required for early NK-cell development in the bone marrow. (A) Flow cytometry of Stat5f/fNcr1-iCreTg mice and littermate controls. Representative dot plots indicate the percentage of gated Lin−CD122+ NKPs (DX5−NK1.1−) and gated Lin−CD122+ mNKs (DX5+NK1.1+) cells. (B) Bar graphs show the percentage of Lin−CD122+ cells, NKPs (DX5− NK1.1−, gated on Lin−CD122+), and mNKs (DX5+ NK1.1+, gated on Lin−CD122+) in the bone marrow (n = 8 STAT5f/f; n = 7 STAT5f/fNcr1-iCreTg, **P = .0014, Mann Whitney U test). (C) Bar graphs show absolute cell number of Lin−CD122+ cells, NKPs, and mNKs in the bone marrow (n = 8 STAT5f/f; n = 7 STAT5f/fNcr1-iCreTg, **P = .0014, Mann Whitney U test). (D) Histograms show the expression of indicated differentiation markers as percentages. (A-D) Data are representative of at least 3 independent experiments. (D) n ≥ 4 per genotype.
Severe impairment of NK cell–dependent, but not T cell–dependent, tumor surveillance in Stat5f/fNcr1-iCreTg mice
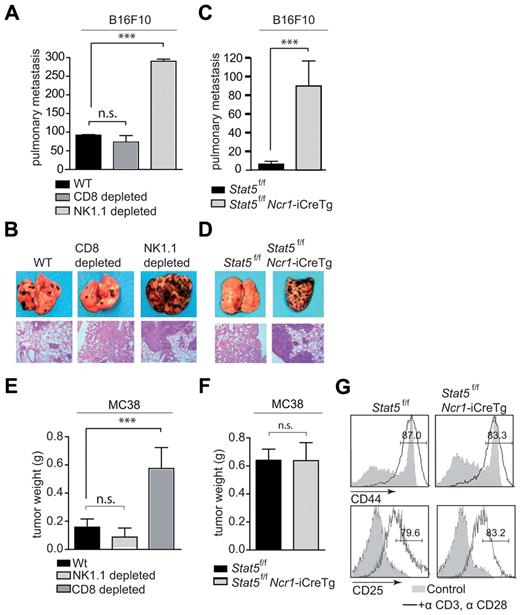
NK cells are well known for their tumor-suppressive role.38,39 To investigate whether the Ncr1-iCreTg–dependent deletion of Stat5 is of functional consequence and affects NK cell–mediated anti-tumor activity, we made use of B16F10 melanoma cells.40 B16F10 cells display low MHC class I levels, indicating a role for NK cells in tumor clearance (supplemental Figure 6). To verify that these cells are indeed exclusively under the tumor surveillance of NK cells, we injected the tumor cell line intravenously into WT mice. These mice were subsequently depleted for either NK cells or cytotoxic T cells using antibodies directed against NK1.1 and CD8, respectively. Twenty-one days later, the experiment was terminated and lung metastases were counted. As depicted in Figure 6A and B, depletion of NK1.1+ cells significantly enhanced the formation of tumor nodules in the lung. In contrast, no differences were observed when CD8+ T cells were depleted compared with the WT. Therefore, we next challenged Stat5f/fNcr1-iCreTg mice and control mice with B16F10 cells. After 12 days, the experiment was terminated. Stat5f/fNcr1-iCreTg mice showed a profound cell infiltration in the lungs. In contrast, only few infiltrating tumor cells were found in the control Stat5f/f animals (Figure 6C-D). These data revealed the strongly impaired NK cell–dependent tumor surveillance in Stat5f/fNcr1-iCreTg mice. We wondered whether this severe defect in NK-cell development and function would affect T cell–mediated tumor surveillance, so we used MC38 adenocarcinoma cells to determine this.41 MC38 tumor cells display high MHC class I levels, pointing to a cytotoxic T-lymphocyte–mediated target-cell recognition (supplemental Figure 6). Indeed, these cells were recognized and lysed by CD8+ cytotoxic T cells, as verified by antibody-dependent depletion of either NK cells or CD8+ T cells (Figure 6E). In this case, only the depletion of CD8+ cells significantly enhanced tumor formation, whereas the repeated application of anti–NK1.1 antibody had no effect compared with the WT. When MC38 cells were subcutaneously injected into Stat5f/fNcr1-iCreTg mice and Stat5f/f littermate controls, we failed to detect any changes in tumor formation between the 2 genotypes (Figure 6F). Similarly, upon T-cell activation in vitro, we did not detect alterations in the expression of activation markers such as CD44 and CD25 on CD3+CD8+ T cells purified from Stat5f/fNcr1-iCreTg versus Stat5f/f mice (Figure 6G).
Tumor surveillance of NK cell–controlled tumors is missing in Stat5f/fNcr1-iCreTg mice. (A-D) B16F10 cells were injected intravenously into (A) WT, WT depleted of CD8+ cells, and WT depleted of NK1.1+ cells and (C) Stat5f/f and Stat5f/fNcr1-iCreTg mice. Numbers of metastatic infiltrates per lung were counted under the binocular microscope after (A) 21 days and (C) 12 days. (B,D) One representative example of an infiltrated lung of the indicated genotype is shown. Top panel: photographs, digital camera, Canon EOS 300D. Bottom panel: H&E-stained histological lung sections; magnification, 100× Zeiss Axiolmager 21, 10× objective, NA 0.25, air; camera: Pixelink Color, 1600 × 1200; software: PixelNK Capture 3.0. (E-F) MC38 cells were injected subcutaneously into (E) WT, WT depleted of CD8+cells, and WT depleted of NK1.1+ cells and (F) Stat5f/f and Ncr1-iCreTg Stat5f/f mice, and after 17 days, tumor weights were analyzed. (G) Histograms showing CD44 (top panel) and CD25 (bottom panel) expression on in vitro–activated T cells from the indicated genotypes. CD8+ T cells were cultured under IL2 and stimulated with plate-bound anti–CD3 + anti–CD28 antibodies. Cells were gated on CD3+CD8+ populations. Open histograms indicate the percentage of CD44+ or CD25+ T cells. Gray histograms indicate unstimulated T cells. (A, C, E, F) n ≥ 5 per genotype. (G) Five mice per genotype were pooled. Data are representative of 3 independent experiments.
Tumor surveillance of NK cell–controlled tumors is missing in Stat5f/fNcr1-iCreTg mice. (A-D) B16F10 cells were injected intravenously into (A) WT, WT depleted of CD8+ cells, and WT depleted of NK1.1+ cells and (C) Stat5f/f and Stat5f/fNcr1-iCreTg mice. Numbers of metastatic infiltrates per lung were counted under the binocular microscope after (A) 21 days and (C) 12 days. (B,D) One representative example of an infiltrated lung of the indicated genotype is shown. Top panel: photographs, digital camera, Canon EOS 300D. Bottom panel: H&E-stained histological lung sections; magnification, 100× Zeiss Axiolmager 21, 10× objective, NA 0.25, air; camera: Pixelink Color, 1600 × 1200; software: PixelNK Capture 3.0. (E-F) MC38 cells were injected subcutaneously into (E) WT, WT depleted of CD8+cells, and WT depleted of NK1.1+ cells and (F) Stat5f/f and Ncr1-iCreTg Stat5f/f mice, and after 17 days, tumor weights were analyzed. (G) Histograms showing CD44 (top panel) and CD25 (bottom panel) expression on in vitro–activated T cells from the indicated genotypes. CD8+ T cells were cultured under IL2 and stimulated with plate-bound anti–CD3 + anti–CD28 antibodies. Cells were gated on CD3+CD8+ populations. Open histograms indicate the percentage of CD44+ or CD25+ T cells. Gray histograms indicate unstimulated T cells. (A, C, E, F) n ≥ 5 per genotype. (G) Five mice per genotype were pooled. Data are representative of 3 independent experiments.
Discussion
In this study, we describe the generation of a novel mouse line expressing Cre recombinase under the control of the Ncr1 promoter. This mouse line expresses the Cre recombinase exclusively in NK cells without affecting other lymphoid compartments, allowing us to delineate the essential and cell-intrinsic role of the transcription factor STAT5 in NK cells.
STAT5 is an important component downstream of cytokines that regulate NK-cell biology, such as IL2, IL7, and IL15. IL2 has been implicated in the regulation of NK-cell activity,42 whereas IL7 was shown to play a critical role in thymic NK-cell development.43 The fact that IL15−/− and IL15Rα−/− mice are largely devoid of peripheral NK cells36,37 suggests that IL15 is a cytokine that is dominant in regulating NK-cell development and homeostasis. The transfer of IL15Rα−/− bone marrow into WT recipients resulted in the rescue of NK cells with greatly reduced expression patterns of the Ly49 receptor repertoire.44 In fact, NK cells do not need self-expression of IL15Rα, but do require IL15Rα expression by accessory cells in their environment that deliver their signals via trans-presentation.45 The role of STAT5 in this cytokine-signaling network remained to be determined.
Deletion of Stat5 in a Ncr1-dependent manner prevents normal NK-cell development in the bone marrow. We can unequivocally rule out any toxic effects of Cre recombinase itself because Ncr1-iCreTg mice display normal NK-cell development and unaltered NK-cell functions. The simplest explanation for this phenotype is that STAT5 represents a critical survival factor. The Ncr1-induced expression of Cre recombinase and the subsequent deletion of Stat5 coincide with the disappearance of the developing NK cells. NCR1 becomes expressed at the iNK-cell stage, when the NK cells start to be greatly reduced in number. The few NK cells present in Stat5f/fNcr1-iCreTg animals represent “escapers” because they display unaltered mRNA levels for Stat5a/b when quantitatively analyzed by real-time PCR. This scenario is also supported by our in vitro observation of mature NK cells purified from Stat5f/f spleens, in which adenovirally mediated Cre expression was incompatible with survival. Whereas Stat5f/f-derived NK cells tolerated expression of the empty vector, the expression of Cre recombinase led to the disappearance of the cells. Similar observations have been made in B-lymphoid cells, in which STAT5 regulates survival during B-cell development.23 STAT5 has also been shown to regulate important anti-apoptotic genes such as Mcl-1, Bcl-xL, and Bcl-2.46 In addition, STAT5 is capable of interfering with and activating the phosphoinositide 3-kinase pathway via growth factor receptor–bound protein 2–associated binding protein 2 (GAB), another signaling pathway important for survival.47
A strong argument against this simplistic view restricting STAT5 to a role as the mediator of survival is the fact that significantly higher numbers of NKPs accumulate in the bone marrow of Stat5f/fNcr1-iCreTg mice. This accumulation is indicative of a developmental block at the NKP stage, and suggests that the decline in STAT5 expression at the transition to the iNK-cell stage is incompatible with further development. The hindered transition into the iNK-cell stage leads to an accumulation of NK cells at the NKP stage. A developmental block is also reflected by the immature nature of bone marrow NK cells, as indicated by the altered expression levels of the activatory receptor Ly49D and the inhibitory receptors Ly49C and Ly49I. The incompleteness of the phenotype, with a reduction but not a complete absence of iNK cells, most likely reflects the long half-life of the STAT5 protein. We have observed that the genomic deletion of Stat5a/b in B-lymphoid cells induces apoptosis only after 7 days due to the slow protein degradation (A. Hoelbl, unpublished data, January 2010).
The molecular mechanisms and transcriptional machinery guiding NK-cell development is largely unknown. Important insights have recently been made by showing the essential functions of the transcription factors ID2 and E4BP4.48-50 The developmental block that we observed in Stat5f/fNcr1-iCreTg mice imitates the phenotype of E4BP4- and IL15R-deficient mice. It is therefore attractive to speculate that this axis, IL15/IL15R-STAT5-E4BP4, determines the fate of NKPs. Further studies are required to delineate the transcriptional network downstream of STAT5 that drives NK-cell development.
The lack of functional NK cells is also confirmed from our in vivo experiments using B16F10 melanoma cells. Tumor nodules in the lung evolve significantly faster in Stat5f/fNcr1-iCreTg mice. It is not manageable to evaluate the cytolytic capacity of Stat5-deficient NK cells because the few NK cells present in Stat5f/fNcr1-iCreTg animals are “escapers” and do express STAT5. Therefore, it is difficult to relate our findings to the report describing a reduced cytolytic response in whole splenocytes derived from Stat5b−/− mice in response to IL2 and IL15.21 These animals lack STAT5B in all cells involved, so any effects are therefore difficult to attribute to a single cell compartment. One might also envision that alterations in cytokine secretion or composition may contribute to these previous observations.
Despite the tight connection between the innate and adaptive arms of the immune system, the severe reduction of NK cells in Stat5f/fNcr1-iCreTg mice did not affect the tumor surveillance of MC38 cells, which is attributed to CD8+ T lymphocytes. Thus, in our experimental setting, the lack of NK cells did not influence the adaptive counterpart in tumor immune surveillance.
Intensive efforts are currently under way to develop inhibitors for STAT proteins to be used in clinical research. STAT3 and STAT5 are prime targets because both are implicated in tumor formation and maintenance and both have been shown to be potent proto-oncogenes.51 The constitutive activation of STAT5 is prominent in hematological malignancies, where it may exert a key role in maintenance of the malignant state.52,53 Blocking STAT5 activation in adult mice is surprisingly well tolerated; we recently observed that deleting Stat5 in adult mice for a period of several weeks was only accompanied by minor side effects, including a decrease in B-cell numbers.54 In these investigations, no information on NK cells was collected. However, based on the results of the present study, we anticipate that an inhibition of STAT5 would be associated with a strong decrease in NK-cell numbers and a severe reduction of NK cell–mediated tumor surveillance. This might be particularly problematic in some cases of leukemia. NK cells are capable of recognizing and eradicating leukemic cells very efficiently, as has been demonstrated in mice and humans.55 They become particularly important for the clearance of residual disease because even after an effective and successful chemotherapy, some leukemic cells remain in the body and have to be cleared by the immune system. Thus, it will be important to evaluate which malignant diseases are subjected to NK cell–mediated tumor surveillance before employing inhibitors directed against STAT5.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to F. Colucci, A. Jamieson, and T. Decker for support and discussions. We thank W. Mikultis for providing Adeno supernatants, W. Ellmeier and J. Raberger for CD1d tetramers, L. Hennighausen for the Stat5f/f mouse, and S. Kawamoto for the eGFPLSL mouse. We thank the Cell Sorting Core Unit of the Medical University Vienna for expert cell sorting.
Financial support for this project was provided by grants from the Austrian Science Foundation (FWF-SFB 28), the Vienna Science and Technology Fund (WWTF-LS07-037), a GENAU grant (Austromouse II, III), and Biomodels Austria.
Authorship
Contribution: E.E., W.W., E.Z., O.S., E.C., and V.S. designed and performed research; E.E., W.W., E.C., and V.S. analyzed data; D.S., T.K., T.R., M.M., and E.C. provided vital new reagents and analytic tools; and E.E. and V.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for O.S. is Immunology Frontier Research Center (IFReC), Osaka University, Osaka, Japan.
Correspondence: Veronika Sexl, Institute of Pharmacology, Währingerstrasse 13A, A-1090 Vienna, Austria; e-mail: veronika.sexl@meduniwien.ac.at.
References
Author notes
E.C. and V.S. contributed equally to this study.

![Figure 2. Development and function is not altered in Cre-expressing NK cells. Neither the development nor the proliferation or cytotoxicity of NK cells was influenced by Cre recombinase. (A) Simplified scheme of NCRI expression in NK-cell development. (B-E) Flow cytometry of Ncr1-iCreTg mice and littermate controls. Dot plots indicate the percentage of (B) gated Lin−CD122+ DX5−NK1.1− NKPs in the BM, (C) gated CD3− NK cells (NK1.1+DX5+) in the periphery, and (D) expression of maturation markers of splenic NK cells gated as TCRβ−NK1.1+ stained with CD27 and CD11b antibodies (n ≥ 4 per genotype). Data are representative of at least 3 independent experiments. (E) In vivo proliferation of splenic NK cells. Mice were injected intraperitoneally with BrdU and after 12 hours, the incorporation of BrdU in splenic NK cells was analyzed. Numbers adjacent to outlined areas in the dot plot indicate the percentage of CD3−NK1.1+ cells. Histograms show the percentage of BrdU-positive cells (n ≥ 5 per genotype). Data are representative of 2 independent experiments. (F) In vitro proliferation of IL2-expanded NK cells purified from the spleens of mice of the indicated genotypes. At day 6, NK cells were seeded in triplicate in 96-well plates. After 12 hours, proliferation was measured by standard 3[H]-thymidine incorporation. Four mice per genotype were pooled. Data are representative of 2 independent experiments. (G) Cytotoxicity of IL2-expanded splenic NK cells purified from indicated genotypes. At day 10, NK cells were coincubated in triplicate with CFSE-labeled YAC-1, RMA-Rae1γ, and RMA targets at the indicated E:T ratios. Five mice per genotype were pooled. Data are representative of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/5/10.1182_blood-2010-06-291633/4/m_zh89991065930002.jpeg?Expires=1769084671&Signature=mU31U-k9S2fVtrkZZRq6w~IFiPWj~Lw1Irdh67DHEZBJG9kuqGf2bnmgPPot9mKeL~c2marZRdefl8Tl15poRZJ4gQGQCkk~tFrOZ7gEUfH~M55N-Tc6v1AhTYgR0cGJP-DlyW~7f-xRTNUvRSQWFOUGqc4ozI6Mbv37HTlwP5fJ4idAMcpb0SsbNLD9w09rI5buD4FvGvDA1QZS5SS3VG7ABK1DARZe2lv97cTO-zurCmRX0Okr9XdXaahPnO5cXHg3iFMkfuVPWGFAz7ySQFdy22hoS8~LizBgiPwJn1MoXPW9Dnbw998khJQpILhr55FJx3YLaT1N~yyS7WmL7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal