Abstract
Levels of regulatory T cells (Tregs) are increased in different cancer types as well as in inflammatory diseases, such as rheumatoid arthritis. Treg accumulation may result from aberrant proliferation and trafficking as well as greater resilience to oxidative stress compared with conventional T cells. This enhanced antioxidative capacity of Tregs possibly serves as feedback inhibition during inflammation and prevents uncontrolled immune reactions by favoring survival of suppressor rather than effector cells. In this study, we demonstrate that human Tregs express and secrete higher levels of thioredoxin-1, a major antioxidative molecule. Thioredoxin-1 has an essential role in maintaining their surface thiol density as the first line of antioxidative defense mechanisms and is sensitive to proinflammatory stimuli, mainly tumor necrosis factor-α, in a nuclear factor-κB-dependent fashion. The antiapoptotic and oncogenic potential of (secreted) Trx-1 suggests that it may exert effects in Tregs beyond redox regulation.
Introduction
Naturally occurring regulatory T cells (Tregs) represent 4% to 5% of the total CD4+ T-cell population. Tregs exhibit strong immunosuppressive ability that is critical for the prevention of autoimmunity, but also hamper efforts to overcome tolerance against tumor antigens. Recently, we described an attribute of human Tregs, which together with peripheral expansion and redirected trafficking may contribute to their increased levels observed typically in cancer1 and inflammation.2,3 Freshly isolated Tregs are resilient to oxidative stress-induced cell death compared with conventional CD4+ T cells (T4conv) and maintain their suppressive function, even at H2O2 dosages lethal for T4conv.4 This increased persistence within inflammatory(-like) environments containing high levels of reactive oxygen species may complement their ability to normally regulate immune responses but also deters therapeutic immune responses in cancer.5,6
Thioredoxin (Trx) is a 12-kDa, ubiquitously expressed enzyme, containing a conserved Trp-Cys-Gly-Pro-Cys-Lys catalytic site. Trx counteracts oxidative stress by scavenging reactive oxygen species and by regulating other enzymes metabolizing H2O2.7 Furthermore, it can act as an antiapoptotic7 and immunomodulating8-10 factor as well as a putative oncogene.11,12
In this study, we demonstrate that human Tregs express and secrete higher levels of Trx-1 than T4conv. Trx-1 is critical for the resilience of Tregs to oxidative stress and sustained expression of surface thiols at a high density.4,5,13 The Trx-1 system is responsive to inflammatory stimuli, mainly tumor necrosis factor-α (TNF-α),2,14-17 and may bolster survival of Tregs in inflammatory environments as part of a negative feedback mechanism during inflammation.
Methods
Cells
This study was approved by the Ethics Committee of the Karolinska University Hospital and performed in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells were isolated by Ficoll-Paque (GE Healthcare). Tregs (CD4+CD25+CD127dim/−), T4conv, and naive/memory subsets were purified immunomagnetically using commercial kits according to the manufacturer's instructions (Miltenyi Biotec).
Antibodies and flow cytometry
Antibodies are detailed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Intracellular redox levels were quantified by labeling with CM-H(2)dichlorodihydrofluorescein diacetate (Invitrogen), and surface thiols were stained with AlexaFluor 633-coupled maleimides (ALM-633; Invitrogen) as described previously.4 Cells were analyzed using an LSRII flow cytometer (BD Biosciences) and FlowJo Version 9.0.2 software (TreeStar).
Cell death assessment
Cell death was determined by 7-amino-actinomycin D (BD Biosciences) and annexin V-fluorescein isothiocyanate (eBioscience) costaining and/or lactate dehydrogenase release assays (Promega). The percentage of specific cell death was calculated according to the following formula:
Specific cell death (% age) = 100 × (% dead cells − % baseline dead cells)/(100% − % baseline dead cells).
Suppression assays
A total of 5 × 104 carboxyfluorescein succinimidyl ester-labeled T4conv were plated alone or together with Tregs in different ratios and stimulated with beads (Treg suppression inspector, Miltenyi Biotec) for 5 days. Proliferation was assessed by flow cytometry by calculating the division index.
Detection of secreted interferon-γ and thioredoxin
Culture supernatants were analyzed by a sandwich enzyme-linked immunosorbent assay (ELISA; R&D Systems) using monoclonal antibodies specific for human interferon-γ (R&D Systems), Trx-1 (IMCO), and Trx80 (IMCO).
RNA preparation and quantitative reverse-transcribed polymerase chain reaction
Total RNA was extracted (RNeasy mini kit; QIAGEN) and cDNA prepared (iScript cDNA synthesis kit; Bio-Rad). Trx-1 mRNA levels were quantified by real-time quantitative polymerase chain reaction (SYBR Green Supermix; ABI 7500 Detection System, Applied Biosystems). Relative gene expression was determined by normalizing the expression to β-actin using specific primers for Trx-1: forward, 5′-ACGCTGCAGGTGATAAAC-3′; reverse, 5′-CTGACAGTCATCCACATCTAC-3′.
Reagents
Auranofin, hydrogen peroxide (H2O2), carboxyfluorescein succinimidyl ester, methylamine, n-ethyl-maleimide (NEM), and PS-1145 dihydrochloride were purchased from Sigma-Aldrich. The recombinant cytokines interferon-γ, interleukin-2 (IL-2), and TNF-α were purchased from R&D Systems.
Statistical analysis
Differences in means were evaluated with parametric (2 tailed student or paired t test) or nonparametric (Mann-Whitney U or Wilcoxon) tests. All analyses were performed using SPSS, Version 16.0.
Results and discussion
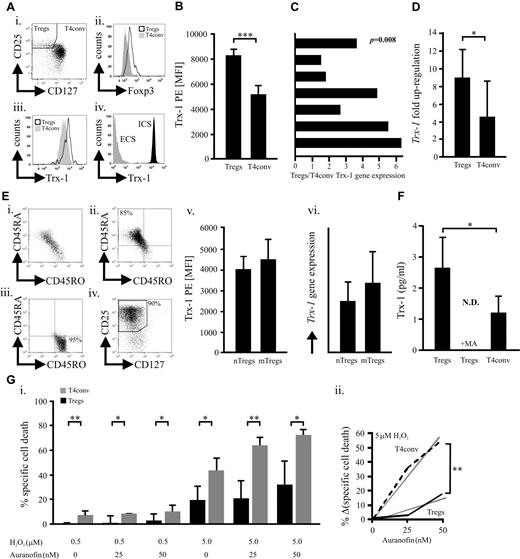
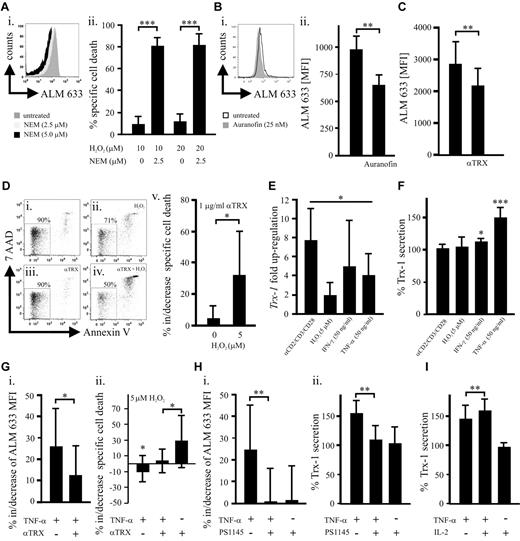
We have previously shown that human naturally occurring CD3+CD4+CD25+CD127low/negativeFOXP3+ Tregs (Figure 1A; supplemental Figure 1) better tolerate oxidative stress than T4conv.4 Their relatively high basal expression of the key antioxidative molecule7,11,12 Trx-1 (Figure 1A-C; supplemental Figure 1) could be one of the underlying causes. Although activated CD25+ T4conv ex vivo exhibit increased Trx-1, levels found in Tregs are significantly higher (supplemental Figure 2). In addition, TCR/CD28 stimulation led to a stronger Trx-1 up-regulation in Tregs (Figure 1D). There was no significant difference regarding Trx-1 expression between naive and memory Tregs, although these subsets exhibit diverse sensitivities toward oxidative stress-induced cell death, analogous to naive and memory T4conv (Figure 1E; supplemental Figure 3).4,6 Normal and malignant cells, including lymphocytes, secrete Trx-1, which can act as an extracellular antioxidant but also in a cytokine-like fashion or even as a putative oncogene.7,8,11,12,18,19 We observed a significantly higher basal Trx-1 secretion by Tregs through a leaderless secretory pathway (Figure 1F),19 whereas truncated Trx was not detectable (data not shown).9,10 During redox reactions, thiols within the catalytic center of Trx-1 form a covalent disulfide bond that is reduced by the Trx-reductase (TrxR) and represents an obligatory step for reestablishing functionality. Inhibition of TrxR and regeneration of functional Trx-1 by sublethal dosages (supplemental Figure 4) of the antirheumatic drug auranofin20 increased the susceptibility of T4conv to H2O2-induced cell death to a greater extent (Figure 1G). This observation suggested that Tregs have a more potent Trx-1 system. Cellular subsets with a superior tolerance toward oxidative stress, such as Tregs, myeloid dendritic cells, and CD56bright NK cells, are characterized by a higher density of surface thiols.4,5,13 Depletion of Treg surface thiols with sublethal doses of NEM21 (supplemental Figure 5) dramatically increased their susceptibility to H2O2-induced cell death (Figure 2A). These results highlight the essential nature of surface thiols in neutralizing reactive oxygen species as the first line of defense. Blocking total and secreted Trx-1 reduced surface thiols (Figure 2B-C) by approximately 30% and approximately 20%, respectively, without affecting intracellular oxidation (supplemental Figure 6). Decrease of surface thiols resulted in increased sensitization to H2O2-induced cell death (Figures 1G, 2D). Inflammatory stimuli with low inherent cytotoxicity (supplemental Figure 7) all led to an up-regulation of Trx-1 expression, but only TNF-α elevated Trx-1 release (Figure 2E-F). Recently, it was described that Tregs express the highest levels of TNFR2 among lymphocytes15-17 (supplemental Figure 10), which unlike TNFR1 does not contain a death domain but rather promotes cell survival and proliferation.2,14 In conjunction with increased expression and secretion of Trx-1 and in contrast to T4conv (supplemental Figure 9), TNF-α enhanced surface thiol expression as well as resilience to H2O2. These effects were antagonized by anti–Trx-1-neutralizing antibodies (Figure 2G). A recent study demonstrated that TNF-α induces the preferential activation of the canonical nuclear factor-κB (NF-κB) pathway in Tregs but not T4conv.22 In keeping with this report, inhibition of NF-κB abolished the TNF-induced increase of surface thiols as well as Trx-1 secretion (Figure 2H). IL-2 and TNF-α act in concert by boosting FOXP3 expression and suppressive activity of murine Tregs.2,14 Treatment of human Tregs with IL-2 resulted in a TNFR2 up-regulation (supplemental Figure 10) and had a synergistic effect on the TNF-α-induced Trx-1 release (Figure 2I). Treg homeostasis at the site of inflammation is regulated by several factors, including lower activation-induced cell death and cytokines (primarily IL-2) attenuating sensitivity to apoptosis.23 Our findings are in accordance with recent observations suggesting that TNF signaling enhances suppressivity of Tregs as part of a negative feedback mechanism during inflammatory processes.2,16,17,24 Moreover, TNF-α, the major inflammatory mediator, promotes survivability of Tregs by bolstering their persistence within an inflammatory, oxygen species-rich milieu as seen in rheumatoid arthritis,3 cancer,1,25 or infections.2,24 Secreted Trx-1 exhibits potential oncogenic effects11,12 as well as immunomodulatory activity (not involving Treg-mediated T-cell suppression, supplemental Figure 11)8 beyond its antioxidative function. Our findings may therefore expand the role of Tregs in cancer, which needs to be further investigated.
Naturally occurring human Tregs express and secrete higher levels of Trx-1 compared with T4conv. (A) Intracellular (ICS) and extracellular (ECS) Trx-1 (iii-iv) was assessed by flow cytometry in freshly purified CD4+ T cells stained with Trx-1 before or after cell permeabilization on gating on CD25+CD127low/negativeFOXP3+ Tregs and CD127positiveFOXP3negative T4conv (i-ii). (B) Trx-1 levels in Tregs and T4conv (n = 9) were quantified by flow cytometry based on the mean fluorescence index (MFI) of Trx-1 PE. (C) Trx-1 relative gene expression was determined in freshly purified Tregs and T4conv (n = 7). Each bar represents the Tregs/T4conv gene expression ratio from one donor. (D) Up-regulation of the Trx-1 relative gene expression was assessed in isolated Tregs and T4conv (n = 4) stimulated for 16 hours with activating anti-CD2, anti-CD3, and anti-CD28 beads. (E) Negatively selected CD4+ T-cells (i) were further subjected to a CD45RA+/CD45RO+ (ii-iii) and subsequent Treg (iv) isolation. (v-vi) MFI of Trx-1 PE and the Trx-1 relative gene expression was assessed in naive CD45RA+ (nTregs) and memory CD45RO+ (mTregs) Treg subsets (n = 6). (F) On 16-hour culture of Tregs and T4conv (n = 6) in AIM-V medium, full-length Trx-1 was quantified in the supernatants by ELISA. Parallel samples were treated overnight with 5mM of methylamine (MA), an inhibitor of the nonclassic, leaderless secretory pathway. (G) Freshly isolated Tregs and T4conv (n = 6) were cultured for 16 hours in the presence of H2O2 (0.5 or 5μM) with or without the TrxR inhibitor auranofin (25 or 50nM). (i) Specific cell death for all conditions was evaluated by flow cytometric analyses. (ii) The relative increase of specific cell death of Tregs/T4conv (n = 6) treated with H2O2 (5μM) on blocking of TrxR. The statistical analysis was performed by analysis of variance testing. Bars represent SD. *P < .05. **P < .01. ***P < .001. N.D. indicates not detected.
Naturally occurring human Tregs express and secrete higher levels of Trx-1 compared with T4conv. (A) Intracellular (ICS) and extracellular (ECS) Trx-1 (iii-iv) was assessed by flow cytometry in freshly purified CD4+ T cells stained with Trx-1 before or after cell permeabilization on gating on CD25+CD127low/negativeFOXP3+ Tregs and CD127positiveFOXP3negative T4conv (i-ii). (B) Trx-1 levels in Tregs and T4conv (n = 9) were quantified by flow cytometry based on the mean fluorescence index (MFI) of Trx-1 PE. (C) Trx-1 relative gene expression was determined in freshly purified Tregs and T4conv (n = 7). Each bar represents the Tregs/T4conv gene expression ratio from one donor. (D) Up-regulation of the Trx-1 relative gene expression was assessed in isolated Tregs and T4conv (n = 4) stimulated for 16 hours with activating anti-CD2, anti-CD3, and anti-CD28 beads. (E) Negatively selected CD4+ T-cells (i) were further subjected to a CD45RA+/CD45RO+ (ii-iii) and subsequent Treg (iv) isolation. (v-vi) MFI of Trx-1 PE and the Trx-1 relative gene expression was assessed in naive CD45RA+ (nTregs) and memory CD45RO+ (mTregs) Treg subsets (n = 6). (F) On 16-hour culture of Tregs and T4conv (n = 6) in AIM-V medium, full-length Trx-1 was quantified in the supernatants by ELISA. Parallel samples were treated overnight with 5mM of methylamine (MA), an inhibitor of the nonclassic, leaderless secretory pathway. (G) Freshly isolated Tregs and T4conv (n = 6) were cultured for 16 hours in the presence of H2O2 (0.5 or 5μM) with or without the TrxR inhibitor auranofin (25 or 50nM). (i) Specific cell death for all conditions was evaluated by flow cytometric analyses. (ii) The relative increase of specific cell death of Tregs/T4conv (n = 6) treated with H2O2 (5μM) on blocking of TrxR. The statistical analysis was performed by analysis of variance testing. Bars represent SD. *P < .05. **P < .01. ***P < .001. N.D. indicates not detected.
Total and secreted Trx-1 is involved in the maintenance of surface thiols and can be regulated by proinflammatory stimuli, mainly TNF-α. (A) This representative (of 4 independent donors) histogram (i) of a flow cytometric analysis represents the assessment of ALM-633-stained surface thiols on purified Tregs treated with the thiol-depleting agent NEM (2.5 or 5μM). (ii) Purified Tregs (n = 4) were treated overnight with H2O2 (10 or 20μM) with or without NEM (2.5μM) followed by flow cytometric evaluation of the specific cell death. (Bi) A representative (of 6 independent donors) histogram of the flow cytometric analysis of surface thiols on purified Tregs treated overnight with auranofin (25nM). (ii) Surface thiols were quantified based on the ALM-633 MFI and compared between treated (25nM auranofin) and untreated Tregs (n = 6). (C) Purified Tregs (n = 10) were cultured overnight with or without 1 μg/mL of a neutralizing anti-Trx-1 antibody (ATRX-03, IMCO) for blocking of secreted Trx-1. Surface thiols were quantified by flow cytometry. (D) A representative (of 6 independent donors) flow cytometric cell death analysis (viable cells are 7-amino-actinomycin D and annexin-V negative) of untreated Tregs (i) as well as cells treated overnight with 5μM H2O2 (ii), 1 μg/mL anti-Trx-1 antibody (iii), or both H2O2 and Trx-1 neutralizing antibody (iv). Specific cell death of Tregs (n = 6) induced by H2O2 with or without 1 μg/mL anti-Trx-1 antibody was quantified (v). (E) Relative gene expression of Trx-1 in purified Tregs (n = 6) was assessed on overnight culture with anti-CD2, anti-CD3, and anti-CD28 beads in a 1:1 cell to bead ratio, 5μM H2O2, 50 ng/mL interferon-γ, or 50 ng/mL TNF-α. The graph represents the ratio of Trx-1 gene expression in stimulated/unstimulated Tregs. (F) Furthermore, secreted Trx-1 was measured in the supernatants of Tregs (n = 7) treated as described in panel E, and results are shown with Trx-1 secretion in untreated cells set as 100%. (G) Purified Tregs were cultured overnight in the presence of 50 ng/mL TNF-α and/or 1 μg/mL anti-Trx-1 antibody. Alterations in (i) surface thiols (n = 7) and (ii) specific cell death (n = 10) induced by H2O2 (5μM) treatment were evaluated by flow cytometry. (H) The role of NF-κB for the TNF-α-mediated effects was evaluated by overnight treatment of Tregs with 50 ng/mL TNF-α with or without 10μM of the NF-κB inhibitor PS1145. The percentage (in relation to untreated Tregs) changes in (i) surface thiols (n = 10) and (ii) Trx-1 secretion (n = 5) were assessed by flow cytometry and ELISA, respectively. (I) Purified Tregs (n = 6) were cultured overnight with 50 ng/mL TNF-α and/or 100 IU/mL IL-2 followed by an ELISA measurement of Trx-1 in the supernatants. Bars represent SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
Total and secreted Trx-1 is involved in the maintenance of surface thiols and can be regulated by proinflammatory stimuli, mainly TNF-α. (A) This representative (of 4 independent donors) histogram (i) of a flow cytometric analysis represents the assessment of ALM-633-stained surface thiols on purified Tregs treated with the thiol-depleting agent NEM (2.5 or 5μM). (ii) Purified Tregs (n = 4) were treated overnight with H2O2 (10 or 20μM) with or without NEM (2.5μM) followed by flow cytometric evaluation of the specific cell death. (Bi) A representative (of 6 independent donors) histogram of the flow cytometric analysis of surface thiols on purified Tregs treated overnight with auranofin (25nM). (ii) Surface thiols were quantified based on the ALM-633 MFI and compared between treated (25nM auranofin) and untreated Tregs (n = 6). (C) Purified Tregs (n = 10) were cultured overnight with or without 1 μg/mL of a neutralizing anti-Trx-1 antibody (ATRX-03, IMCO) for blocking of secreted Trx-1. Surface thiols were quantified by flow cytometry. (D) A representative (of 6 independent donors) flow cytometric cell death analysis (viable cells are 7-amino-actinomycin D and annexin-V negative) of untreated Tregs (i) as well as cells treated overnight with 5μM H2O2 (ii), 1 μg/mL anti-Trx-1 antibody (iii), or both H2O2 and Trx-1 neutralizing antibody (iv). Specific cell death of Tregs (n = 6) induced by H2O2 with or without 1 μg/mL anti-Trx-1 antibody was quantified (v). (E) Relative gene expression of Trx-1 in purified Tregs (n = 6) was assessed on overnight culture with anti-CD2, anti-CD3, and anti-CD28 beads in a 1:1 cell to bead ratio, 5μM H2O2, 50 ng/mL interferon-γ, or 50 ng/mL TNF-α. The graph represents the ratio of Trx-1 gene expression in stimulated/unstimulated Tregs. (F) Furthermore, secreted Trx-1 was measured in the supernatants of Tregs (n = 7) treated as described in panel E, and results are shown with Trx-1 secretion in untreated cells set as 100%. (G) Purified Tregs were cultured overnight in the presence of 50 ng/mL TNF-α and/or 1 μg/mL anti-Trx-1 antibody. Alterations in (i) surface thiols (n = 7) and (ii) specific cell death (n = 10) induced by H2O2 (5μM) treatment were evaluated by flow cytometry. (H) The role of NF-κB for the TNF-α-mediated effects was evaluated by overnight treatment of Tregs with 50 ng/mL TNF-α with or without 10μM of the NF-κB inhibitor PS1145. The percentage (in relation to untreated Tregs) changes in (i) surface thiols (n = 10) and (ii) Trx-1 secretion (n = 5) were assessed by flow cytometry and ELISA, respectively. (I) Purified Tregs (n = 6) were cultured overnight with 50 ng/mL TNF-α and/or 100 IU/mL IL-2 followed by an ELISA measurement of Trx-1 in the supernatants. Bars represent SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
In conclusion, our data demonstrate that increased Trx-1 is an important contributor to reduced sensitivity of Tregs toward oxidative stress.4 Furthermore, it constitutes, together with inflammatory stimuli, especially TNF-α, a dynamic negative feedback mechanism that promotes Tregs within inflammatory milieus to prevent prolonged or excessive immune responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Raja Choudhury for critical review of the manuscript.
This work was supported by the Cancer Society of Stockholm, Karolinska Institutet, Stockholm City Council, Swedish Cancer Society, and Swedish Research Council. D.M. was supported by the German Research Foundation.
Authorship
Contribution: D.M. designed and performed research, analyzed data, and wrote the manuscript; C.C.J. designed and performed research; R.J. and M.B. performed research; and R.K. designed research and supervised data analysis and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dimitrios Mougiakakos, Department of Oncology and Pathology, Karolinska University Hospital, Cancer Centre Karolinska R8:01, 171 76 Stockholm, Sweden; e-mail: Dimitrios.Mougiakakos@ki.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal