Abstract
Cluster of differentiation (CD)8+ T cells exist as naive, central memory, and effector memory subsets, and any of these populations can be genetically engineered into tumor-reactive effector cells for adoptive immunotherapy. However, the optimal subset from which to derive effector CD8+ T cells for patient treatments is controversial and understudied. We investigated human CD8+ T cells and found that naive cells were not only the most abundant subset but also the population most capable of in vitro expansion and T-cell receptor transgene expression. Despite increased expansion, naive-derived cells displayed minimal effector differentiation, a quality associated with greater efficacy after cell infusion. Similarly, the markers of terminal differentiation, killer cell lectin-like receptor G1 and CD57, were expressed at lower levels in cells of naive origin. Finally, naive-derived effector cells expressed higher CD27 and retained longer telomeres, characteristics that suggest greater proliferative potential and that have been linked to greater efficacy in clinical trials. Thus, these data suggest that naive cells resist terminal differentiation, or “exhaustion,” maintain high replicative potential, and therefore may be the superior subset for use in adoptive immunotherapy.
Introduction
It is now possible to genetically engineer human T lymphocytes to express virtually any known gene, including genes encoding T-cell receptors (TCRs) or chimeric antigen receptors (CARs), to provide the desired T-cell specificity. These gene-engineered lymphocytes hold the promise to treat infectious diseases and cancer,1-10 but the appropriate substrate cells to use is understudied, and most current protocols simply transfer genes into “bulk” peripheral blood mononuclear cells (PBMCs). Varieties of data indicate that T cells, and in particular CD8+ T cells, are worthy substrates for gene engineering. However, CD8+ T cells in peripheral blood are themselves a complex mixture, composed of at least 3 major subsets—naive (TN), central memory (TCM), and effector memory (TEM)—each having different functional qualities.
The optimal subset to engineer for adoptive immunotherapy is controversial.8,11-14 Memory CD8+ T-cell subsets are more studied than naive cells, because antigen-specific clones can be found at increased frequencies. In a landmark paper, Berger et al13 have shown in macaques that effector cells derived from TCM rather than TEM possess greater ability to survive and establish immunologic memory after infusion. This finding is consistent with data comparing memory subsets in mice.15 However, TN cells were not explicitly analyzed in these reports,12 an omission that might be important given recent findings in mice that naive T cells convey more antitumor activity than memory cells.12
Short of conducting a series of clinical trials, the decision of which CD8+ T-cell subset to use for clinical protocols will depend on the cell phenotypes that result from transduction of T-cell subsets. Recent efforts have therefore focused on identifying traits of T cells in vitro that correlate with increased effectiveness in vivo. Both human and animal studies indicate that effective cells for adoptive immunotherapy must possess proliferative potential and the capacity to acquire and maintain effector function after infusion.9 Analyses of tumor-infiltrating lymphocytes before infusion have revealed phenotypic qualities that correlate with objective responses in patients with metastatic melanoma. Specifically, T cells associated with clinical efficacy have higher levels of the costimulatory molecule CD27 and longer telomeres.16-18 Studies performed in mouse models have revealed that optimal T cells for therapy display low levels of eomesodermin (Eomes), a T-box transcription factor that promotes full effector function in CD8+ T cells.12,19-21 Concordantly, diminished interferon-γ (IFN-γ) production and minimal specific cytolysis are also associated with more efficacious cells.12,19,21 Therapeutically effective cells in mouse models also express low levels of killer cell lectin-like receptor G1 (KLRG-1), a molecule implicated in T-cell exhaustion and replicative senescence.12,19,21,22 Taken together, this work has led to the realization that, counterintuitively, development of effector cell qualities in vitro results in impaired antitumor function in vivo, a finding attributed to the decreased proliferative potential of more differentiated effector cells.9,12,19,20,23
We set out to study human T cells derived from TN, TCM, and TEM subsets to determine which subset possessed the functional and phenotypic characteristics associated with efficacious T cells. We found that effector cells derived from the TN subset displayed minimal effector differentiation and had the lowest levels of markers of terminal differentiation, including KLRG1 and CD57. Naive-derived effector cells also expressed higher CD27 and retained longer telomeres. In addition, we found that the use of TN cells rather than TCM or TEM cells as a cellular substrate for TCR gene engineering had the practical advantages of increased transgene expression and more robust proliferation. Taken together, these findings indicate that adoptive immunotherapy with T cells expressing TCR or CAR transgenes might be improved by transducing purified TN cells.
Methods
T-cell isolation and cell lines
Cryopreserved leukapheresis samples from patients with metastatic melanoma on National Cancer Institute board-approved clinical trials were used for all studies except Figure 1A, which used samples from 11 healthy donors. Cryopreserved cell vials were selected based on recent freeze date and availability of sufficient numbers of cells for the assays. In all experiments, cells were initially thawed and rested overnight in culture media at 37°C. CD8+ magnetic bead enrichment was then performed (StemCell Technologies). The cells were then labeled with anti-CD8, anti-CD62L, and anti-CD45RO fluorescent antibodies (BD) and fluorescence-activated cell sorted (FACS) into TN, TCM, and TEM subsets on a BD FACSAria sorter (BD Biosciences). The previously described NY-ESO-1+ HLA-A2+ tumor lines, 624.38 and 1359A2, served as tumor targets in some assays.24,25 An NY-ESO-1− HLA-A2+ renal cell carcinoma line, 2661R, was obtained from a Surgery Branch patient.25
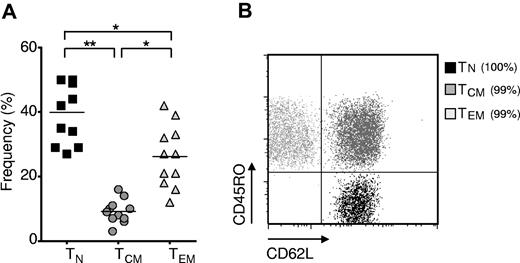
Naive CD8+ T cells are the most abundant subset of human CD8+ T cells. Cryopreserved leukapheresis samples were thawed and rested overnight. (A) Frequency of TN (CD62L+, CD45RO−), TCM (CD62L+, CD45RO+), and TEM (CD62L−, CD45RO+) CD8+ T-cell subsets in leukapheresis samples from 11 healthy adult donors (*P < .01, **P < .001). (B) Representative flow cytometric dot plot with overlay of FACS-sorted cells from the TN, TCM, and TEM subsets. The purity of each subset is indicated in parentheses. The dot plot is on log axes.
Naive CD8+ T cells are the most abundant subset of human CD8+ T cells. Cryopreserved leukapheresis samples were thawed and rested overnight. (A) Frequency of TN (CD62L+, CD45RO−), TCM (CD62L+, CD45RO+), and TEM (CD62L−, CD45RO+) CD8+ T-cell subsets in leukapheresis samples from 11 healthy adult donors (*P < .01, **P < .001). (B) Representative flow cytometric dot plot with overlay of FACS-sorted cells from the TN, TCM, and TEM subsets. The purity of each subset is indicated in parentheses. The dot plot is on log axes.
Cell stimulation and transduction
Assays for transgene expression, culture expansion, phenotype, gene expression, telomere length, and function were performed as follows: Isolated CD8+ T-cell subsets were stimulated with irradiated (50 Gy) allogeneic peripheral blood mononuclear cells at a 10:1 ratio in the presence of soluble anti-CD3 (OKT3) 30 ng/mL and interleukin 2 (IL-2) 300 IU/mL. Transduction was performed 48 hours after initial stimulation using retrovirus encoding the 1G4-α95:LY mutant TCR targeting NY-ESO-1.25 Retrovirus was generated from a previously described producer line.26 For the transductions, retroviral supernatant was centrifuged onto 24-well Retronectin-coated (Takara Bio) plates for 2 hours at 2000g and 32°C. CD8+ T cells were then plated and centrifuged for 10 minutes at 1500 rpm. After approximately 16 hours, the cells were moved from Retronectin-coated plates to 24-well tissue culture-treated plates (Corning). Cells were cultured for a total of 10 to 16 days before assays (ie, 8-14 days after completion of the transduction).
Proliferation and cell death assays
Isolated T-cell subsets were plated in a 1:1 ratio with Dynabeads Human T-Activator CD3/CD28 (Invitrogen) in culture media with IL-2 300 IU/mL. Dynabeads were removed with magnets after 48 hours of stimulation. For the H3 -TdR incorporation assays, cells were pulsed with 1 μCi H3 -TdR 48 hours after initial stimulation then incubated for 16 hours. Samples were harvested using a semiautomated sample harvester, and counts per minute were determined with a β-scintillation counter. Carboxyfluorescein succinimidyl ester (CFSE) studies were performed by labeling cells with 1μM CFSE (Invitrogen) before stimulation. Three days later, CFSE dilution was determined by flow cytometry and analyzed using FlowJo Version 7.5 software (TreeStar). Annexin V/7-AAD assays were performed on cells after overnight stimulation using a commercially available kit (BD Pharmingen).
Flow cytometry, cytokine production assays, cytolytic assays, and gene expression analysis
Cells were labeled with fluorescent antibodies against the following targets: CD8, CD62L, CD45RO, CD27, and CD57 (all from BD Biosciences); CCR7 (R&D Systems); and Vβ13.1 (Beckman Coulter). NY-ESO-1-HLA-A2 tetramer was manufactured at the National Institutes of Health Tetramer Core Facility in Atlanta, GA. Intracellular IFN-γ levels were determined by coculture of transduced effector cells with the 624.38 (NY-ESO-1+ HLA-A2+) cell line in the presence of brefeldin A (BD Biosciences). The cell surface was labeled with anti-CD8 and cells were fixed and permeabilized with BD Cytofix/Cytoperm (BD Biosciences). Intracellular IFN-γ labeling was performed according to the manufacturer instructions (BD Biosciences). Flow cytometric data were acquired using a BD FACSCanto II cytometer (BD Biosciences) and were analyzed with FlowJo Version 7.5 software (TreeStar). Flow cytometric dot plots are shown with log scale axes. Histograms are on a log scale x-axis and a linear y-axis. For cytokine production assays, transduced effector cells were cocultured overnight with 624.38 and/or 1359A2 (NY-ESO-1+ HLA-A2+) cells or 2661R (NY-ESO-1− HLA-A2+) cells. IFN-γ levels in the supernatants were determined by enzyme-linked immunosorbent assay (ELISA; R&D Systems). Cytolytic assays were performed as previously described.25 For real-time reverse-transcription–polymerase chain reaction (RT-PCR), RNA was extracted with RNeasy Kits (QIAGEN). cDNA was generated using High Capacity RNA-to-cDNA Kits (Applied Biosystems). Real time RT-PCR was performed on an ABI 7500 Fast Real-Time PCR system (Applied Biosystems) using EOMES, KLRG1, and ACTB primer/probe sets (Applied Biosystems). Expression of EOMES and KLRG1 was determined relative to ACTB. Due to variability in the relative expression values between patients and between independently performed experiments from separate days, the relative expression for each gene was normalized to the average expression for each patient.
Telomere length measurement
Telomere length was determined by quantitative flow-fluorescent in-situ hybridization (flow-FISH).18,27 A fluorescein isothiocyante (FITC)–conjugated telomere probe was purchased from Dako. The 1301 subline of human T-cell lymphoblast-like cell line (CCRF-CEM) was used as a telomere length control (Sigma-Aldrich). Due to variation between patients in prestimulation telomere length, telomere length for each subset was normalized to the mean telomere length for each patient (telomere index = telomere length for subset / average telomere length for patient).
Statistical analysis
Groups were compared using a repeated measures 1-way analysis of variance with a Bonferroni posttest. Error bars represent the SEM.
Results
Greater abundance, expansion, and transduction of naive cells
We sought to assess the relative frequency of postthymic human CD8+ T-cell subsets by determining the frequency of CD62Lhigh CD45ROlow (TN), CD62Lhigh CD45ROhigh (TCM), and CD62Llow CD45ROhigh (TEM) subsets in the leukapheresis samples of eleven normal donors (Figure 1A). Consistent with results from other laboratories,28 we observed an abundance of TN cells in normal healthy donors, although there was significant patient-to-patient variability in T-cell subsets. We found that FACS sorting (Figure 1B) resulted in separation of populations that possessed the functional characteristics classically ascribed to the TN, TCM, and TEM subsets (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).29 We did not isolate the subset of CD45RA+ CD45RO− CCR7− CD62L− CD8+ cells known as T effector memory CD45RA+ (EMRA), thought to be a terminally differentiated CD8+ T-cell population.29
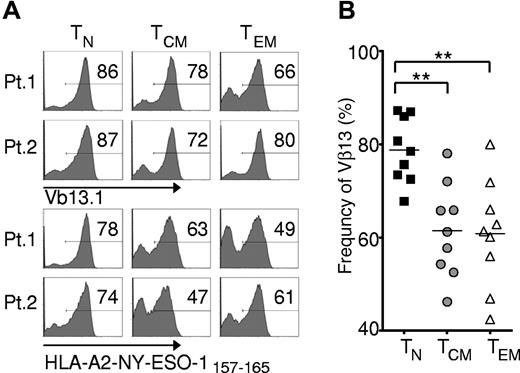
To test the relative frequency of transgene expression of these T-cell subsets, we used a retroviral vector encoding the 1G4-α95:LY mutant TCR, presently employed in a clinical trial being conducted at the National Institutes of Health Clinical Center (Bethesda, MD) that targets an HLA-A*0201-restricted epitope from NY-ESO-1 (amino acid residues 157-165).25 We found that all subsets were efficiently transduced, but the TN-derived subset demonstrated significantly greater frequency of MHC-peptide tetramer binding and expression of the TCR variable β chain encoded by the vectors (Figure 2A-B), with mean transgene expression frequencies approaching 80%.
Effector cells derived from naive rather than memory precursors express the genetically engineered TCR with greater frequency. Cryopreserved leukapheresis samples were thawed and rested overnight. CD8+ T-cell subsets were isolated then stimulated with allogeneic feeders, anti-CD3, and IL-2. Cells were transduced to express the 1G4-α-95:LY mutant TCR 48 hours after stimulation, and flow cytometric analysis was performed 10-16 days after stimulation. (A) Expression of Vβ13.1 (the 1G4 β-chain clonotype) and binding to HLA-A2-NY-ESO-1157-165 tetramer by transduced cells from 2 representative patients. Gate frequencies are displayed. (B) Frequency of Vβ13.1+ cells following transduction of samples from 9 patients (**P < .001).
Effector cells derived from naive rather than memory precursors express the genetically engineered TCR with greater frequency. Cryopreserved leukapheresis samples were thawed and rested overnight. CD8+ T-cell subsets were isolated then stimulated with allogeneic feeders, anti-CD3, and IL-2. Cells were transduced to express the 1G4-α-95:LY mutant TCR 48 hours after stimulation, and flow cytometric analysis was performed 10-16 days after stimulation. (A) Expression of Vβ13.1 (the 1G4 β-chain clonotype) and binding to HLA-A2-NY-ESO-1157-165 tetramer by transduced cells from 2 representative patients. Gate frequencies are displayed. (B) Frequency of Vβ13.1+ cells following transduction of samples from 9 patients (**P < .001).
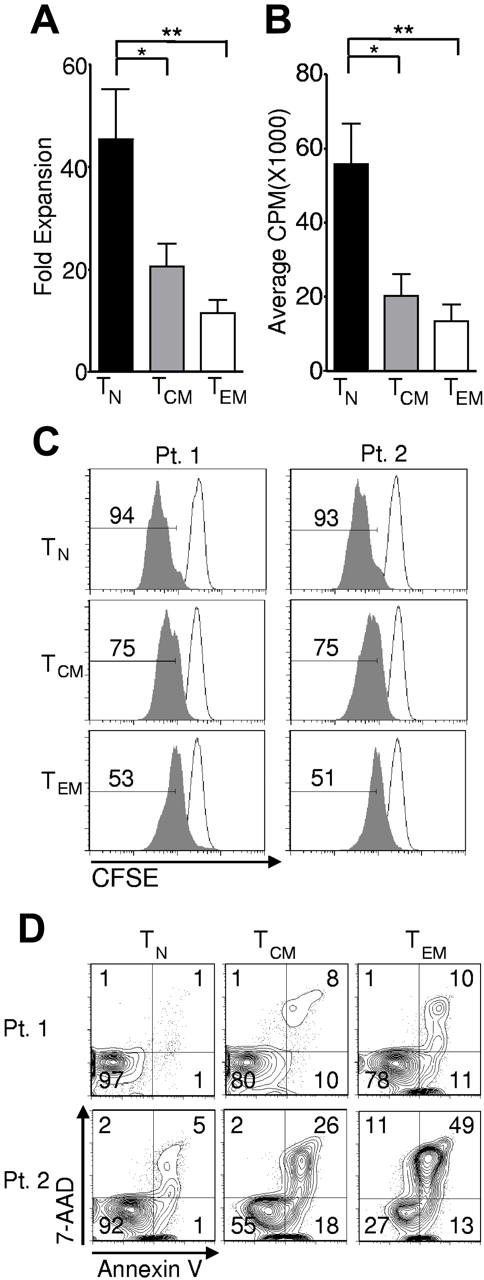
In vivo expansion of T cells might be required to effect the destruction of large amounts of tumor, and the proliferative capacity of adoptively transferred cells correlates with their antitumor effectiveness in the pmel-1 mouse model.15,30,41 We observed significantly greater expansion in cells derived from the TN compared with cells derived from TCM and TEM, as assessed by enumerating the cells after stimulation with allogeneic feeders, anti-CD3, and IL-2 and calculating the fold expansion in their numbers (Figure 3A). To study the relative contributions of cell division and cell death to the increased numbers of TN-derived, transduced T cells, we employed a tritiated thymidine incorporation assay (Figure 3B), which is an estimate of net DNA synthesis. We also assessed cell division by tracking dilution of CFSE (Figure 3C). Both tests indicated enhanced proliferation of the TN population. We sought to assess the relative contribution of apoptosis to the increased cell numbers observed. Although we detected considerable patient-to-patient variability, we measured increases in both early and late apoptosis, as measured by annexin V and 7-AAD, respectively, in the memory populations and particularly in TEM-derived cells (Figure 3D). Thus, enhanced expansion of TN cells was driven by both increased proliferation and by decreased apoptotic death. Taken together, these data showed that TN cells were the most abundant, the most receptive to expression of the transgene, and the most proliferative CD8+ T-cell subset in the peripheral blood of adult humans.
Naive cells display greater in vitro expansion than memory cells. Cryopreserved leukapheresis samples were thawed and rested overnight. CD8+ T-cell subsets were isolated then stimulated with allogeneic feeders, anti-CD3, and IL-2 (A) or stimulated with CD3/CD28 beads and IL-2 (B-C), then transduced to express the 1G4-α-95:LY mutant TCR 48 hours after stimulation. (A) Fold expansion of each culture was determined 10-16 days following stimulation. The fold expansion of CD8+ T-cell subsets from 12 patients is displayed (*P < .01, **P < .001). (B) Cells were pulsed with H3 -TdR 48 hours after stimulation, and incorporation as determined 16 hours later is shown. N = 6. (*P < .01, **P < .001). (C) Cell division was determined by CFSE dilution 3 days after stimulation (shaded histograms). The frequency of cells within each gate is indicated. Open histograms are undivided controls. The x-axis is on a log scale and the y-axis is on a linear scale. Data are representative of 4 patients. (D) One day after transduction, cells were labeled with 7AAD and annexin V and flow cytometry was performed. Gate frequencies are displayed. Results are representative of 6 patients.
Naive cells display greater in vitro expansion than memory cells. Cryopreserved leukapheresis samples were thawed and rested overnight. CD8+ T-cell subsets were isolated then stimulated with allogeneic feeders, anti-CD3, and IL-2 (A) or stimulated with CD3/CD28 beads and IL-2 (B-C), then transduced to express the 1G4-α-95:LY mutant TCR 48 hours after stimulation. (A) Fold expansion of each culture was determined 10-16 days following stimulation. The fold expansion of CD8+ T-cell subsets from 12 patients is displayed (*P < .01, **P < .001). (B) Cells were pulsed with H3 -TdR 48 hours after stimulation, and incorporation as determined 16 hours later is shown. N = 6. (*P < .01, **P < .001). (C) Cell division was determined by CFSE dilution 3 days after stimulation (shaded histograms). The frequency of cells within each gate is indicated. Open histograms are undivided controls. The x-axis is on a log scale and the y-axis is on a linear scale. Data are representative of 4 patients. (D) One day after transduction, cells were labeled with 7AAD and annexin V and flow cytometry was performed. Gate frequencies are displayed. Results are representative of 6 patients.
Resistance of naive-derived effector cells to terminal differentiation
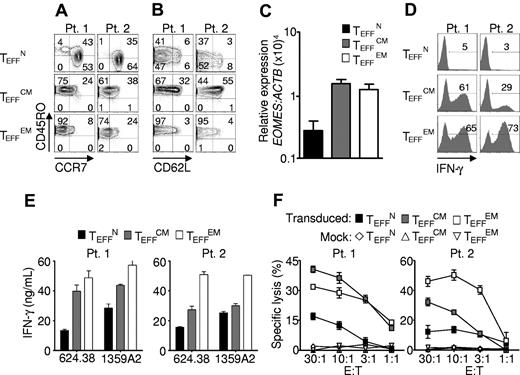
We next examined the phenotypic and functional characteristics of the effector cells generated from each subset. Human CD8+ T-cell subsets were isolated from cryopreserved patient samples, transduced, and expanded for 10 to 16 days, then assessed for the surface expression of CD45RO, CD62L, and CCR7. Despite their increased expansion (Figure 3A-C), effector cells from naive progenitors (TEFFN) expressed lower levels of CD45RO than effector cells from central memory (TEFFCM) or effector memory (TEFFEM) progenitors (Figure 2A).29 CCR7 was retained by TEFFN cells, but it was mostly lost by TEFFCM (Figure 4A). This pattern was in contrast to that of CD62L expression, which was better maintained by TEFFCM rather than TEFFN, consistent with mouse studies (Figure 4B).12 These findings indicated that the effector cell phenotype was influenced by the subset from which the cells were derived.
Effector cells derived from naive precursors manifest less acquisition of effector function. Cryopreserved leukapheresis samples were thawed and rested overnight. CD8+ T-cell subsets were isolated then stimulated with allogeneic feeders, anti-CD3, and IL-2. Cells were transduced to express the 1G4-α-95:LY mutant TCR 48 hours after stimulation. The following data were obtained 10-16 days after stimulation. (A-B) Expression of CD45RO, CD62L, and CCR7. Gate frequencies are indicated. Data are representative of 6 patients. (C) EOMES expression by real-time RT-PCR. Expression is relative to ACTB then normalized to the average relative expression for each patient. Data from 5 patients are shown. (D-E) IFN-γ production from 16-hour coculture with NY-ESO-1+ HLA-A2+ tumor targets as determined by intracellular staining and supernatant concentrations, respectively. Coculture with 2361R (NY-ESO-1− HLA-A2+) results in < 0.2 ng/mL of IFN-γ production in all groups. (F) Specific cell killing as determined by 51Cr release assay against the 624.38 tumor line.
Effector cells derived from naive precursors manifest less acquisition of effector function. Cryopreserved leukapheresis samples were thawed and rested overnight. CD8+ T-cell subsets were isolated then stimulated with allogeneic feeders, anti-CD3, and IL-2. Cells were transduced to express the 1G4-α-95:LY mutant TCR 48 hours after stimulation. The following data were obtained 10-16 days after stimulation. (A-B) Expression of CD45RO, CD62L, and CCR7. Gate frequencies are indicated. Data are representative of 6 patients. (C) EOMES expression by real-time RT-PCR. Expression is relative to ACTB then normalized to the average relative expression for each patient. Data from 5 patients are shown. (D-E) IFN-γ production from 16-hour coculture with NY-ESO-1+ HLA-A2+ tumor targets as determined by intracellular staining and supernatant concentrations, respectively. Coculture with 2361R (NY-ESO-1− HLA-A2+) results in < 0.2 ng/mL of IFN-γ production in all groups. (F) Specific cell killing as determined by 51Cr release assay against the 624.38 tumor line.
To further assess effector CD8+ T-cell development, we examined acquisition of the effector cell transcriptional program. Expression of EOMESODERMIN (EOMES), the transcription factor that confers cytolytic qualities to the natural killer (NK) cell and CD8+ T-cell lineages,31 was markedly lower in TEFFN rather than in TEFFCM and TEFFEM (Figure 4C). We found that this diminished expression of EOMES correlated with reduced release of IFN-γ (Figure 4D-E) and with lesser specific cell killing (Figure 4F). These findings are consistent with those in mice indicating that Eomes, a paralogue of Tbet (Tbx21), drives full effector differentiation in CD8+ T cells after activation.31
Lower levels of IFN-γ release and reduced levels of cytotoxicity are traits that are associated with greater antitumor function following adoptive transfer in a variety of settings. These include multiple stimulated cells,30 IL-21– and IL-15–primed cells,23,32 WNT signal–programmed cells,20 type 17 CD8+ T cells (Tc17),21 and naive-derived murine effector CD8+ T cells.12 In mouse models it is critically important that T cells be able to acquire effector functions (such as the ability to produce IFN-γ) in order to have antitumor efficacy.33 In the present studies, we indeed found that TEFFN cells did acquire full cytolytic function with additional stimulation, demonstrating that their potential to develop into full effector cells in this setting was not impaired (supplemental Figure 2). Taken together, these results indicated that cells of naive origin showed diminished effector cell differentiation, suggesting less progress toward terminal differentiation.
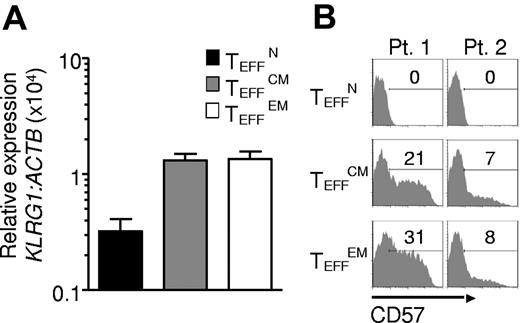
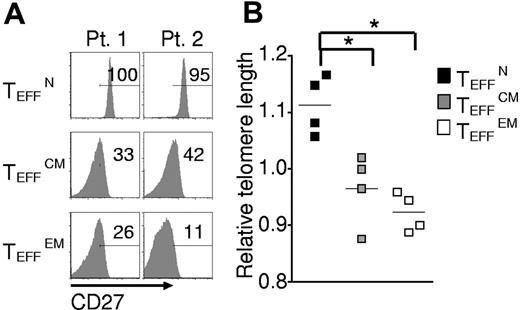
To additionally test for terminal differentiation, killer cell lectin-like receptor G1 (KLRG1) and beta-1, 3-glucoronyltransferase 1 (B3GAT1) encoding CD57 expression were analyzed. In mice, Klrg1 is expressed by terminally differentiated effector cells generated in vitro23,30 and by effector cells destined for short life in vivo.34 Similarly, in humans KLRG1 and CD57 are found on the surfaces of senescent effector cells induced by chronic infection.30 KLRG1 and CD57 expression was lowest in TEFFN cells, which displayed low KLRG1 and did not express detectable CD57 (Figure 5A-B). To further study the proliferative potential of the effector cells, we analyzed CD27 expression and telomere length, qualities that positively correlate with replicative capacity and tumor response in clinical trials.16,18 CD27 expression was almost uniformly high in TEFFN cells (Figure 6A) and lower in TEFFCM and TEFFEM cells (Figure 6B). Similarly, telomere length was greatest in the effector cells derived from naive cells (TEFFN; Figure 6B), a finding consistent with previously published work demonstrating that human naive T cells possess longer telomeres before stimulation.35,36 These findings indicated that naive-derived effector cells, despite their increased proliferation in vitro, resisted terminal differentiation and retained the characteristics of effective cells for adoptive immunotherapy.
Naive-derived effector cells demonstrate resistance to terminal differentiation. Cryopreserved leukapheresis samples were thawed and rested overnight. CD8+ T-cell subsets were isolated then stimulated with allogeneic feeders, anti-CD3, and IL-2. Cells were transduced to express the 1G4-α-95:LY mutant TCR 48 hours after stimulation. The following data were obtained 10-16 days after stimulation. (A) KLRG1 expression by real time RT-PCR. Expression is relative to ACTB then normalized to the average relative expression for each patient. Data from 5 patients are shown. (B) Flow cytometric analysis of CD57 expression. Gate frequencies are indicated. Data shown are representative of 3 patients.
Naive-derived effector cells demonstrate resistance to terminal differentiation. Cryopreserved leukapheresis samples were thawed and rested overnight. CD8+ T-cell subsets were isolated then stimulated with allogeneic feeders, anti-CD3, and IL-2. Cells were transduced to express the 1G4-α-95:LY mutant TCR 48 hours after stimulation. The following data were obtained 10-16 days after stimulation. (A) KLRG1 expression by real time RT-PCR. Expression is relative to ACTB then normalized to the average relative expression for each patient. Data from 5 patients are shown. (B) Flow cytometric analysis of CD57 expression. Gate frequencies are indicated. Data shown are representative of 3 patients.
Naive-derived effector cells display indicators of enhanced proliferative potential and efficacy. Cryopreserved leukapheresis samples were thawed and rested overnight. CD8+ T-cell subsets were isolated then stimulated with allogeneic feeders, anti-CD3, and IL-2. Cells were transduced to express the 1G4-α-95:LY mutant TCR 48 hours after stimulation. The following data were obtained 10-16 days after stimulation. (A) Expression of CD27 as determined by flow cytometry. Gate frequencies are shown. Data are representative of 6 patients. (B) Telomere length relative to average length for each patient. Four individual patients are represented by different shapes (*P < .05).
Naive-derived effector cells display indicators of enhanced proliferative potential and efficacy. Cryopreserved leukapheresis samples were thawed and rested overnight. CD8+ T-cell subsets were isolated then stimulated with allogeneic feeders, anti-CD3, and IL-2. Cells were transduced to express the 1G4-α-95:LY mutant TCR 48 hours after stimulation. The following data were obtained 10-16 days after stimulation. (A) Expression of CD27 as determined by flow cytometry. Gate frequencies are shown. Data are representative of 6 patients. (B) Telomere length relative to average length for each patient. Four individual patients are represented by different shapes (*P < .05).
Discussion
Adoptive cell transfer-based immunotherapy using T lymphocytes transduced with TCR or CAR is a promising approach to cancer treatment. Recent advances in host preparation are highly effective in mouse models37 and may increase the objective response rate in patients with metastatic melanoma.10 However, complete and durable tumor regression or “cure” rates must be improved.10,11 It is increasingly evident that cell therapy might be enhanced by the infusion of less-differentiated CD8+ T cells that possess greater proliferative potential.15,19,20,32 In the present manuscript, we report that naive CD8+ T cells, the most common subset in adult humans, expanded more robustly and expressed an engineered transgene more frequently than their memory cell counterparts, thus making them more desirable for clinical use. Furthermore, they displayed the kind of resistance to terminal differentiation and high proliferative potential that are beneficial in adoptive immunotherapy. Thus, naive cells might be the preferable effector cell platform for T-cell transfer-based immunotherapy.
It is important to point out that we have not here tested an in vivo end point that measures the efficacy of the TN-derived cells. The subject of identification of the optimal cell for adoptive transfer has been recently reviewed by Brenner and Heslop.11 A definitive answer to whether use of naive CD8+ T cells would improve adoptive immunotherapy will likely require a clinical trial. An additional caveat to our study is that only a single system of cell transduction and expansion was tested. It is possible that other methods of cell culture (eg, use of IL-7, IL-15, artificial antigen-presenting cells,11,14,38 or stimulatory beads) would give different results. In addition, targeting of memory cells that are capable of being activated by other antigens in the donor (for example, anti-CMV or anti-EBV memory cells), perhaps containing both CD8+ and CD4+ cells, could have a number of advantages for memory substrate cells.1,11,39
Given these caveats, in vivo experiments in mice indicate superiority of naive-derived cells over their memory-derived counterparts in a direct comparison,12 and this study supports that conclusion. We can only conclude that animal studies and retrospectively analyzed clinical trials have yielded valuable clues that may indicate cell characteristics desirable in the cell preparation (less effector differentiation, greater expression of CD27, and longer telomeres) and that naive-derived effector cells more closely approximate these predictors of efficacy.
Another hurdle for the successful translation of any approach utilizing naive T cells includes the impact of aging and prior chemotherapy on the size and robustness of the TN-cell precursor pool. From a practical standpoint, the frequency of naive T cells is highly variable in normal controls (Figure 1A). Patients with cancer are likely to have even more variable levels of TN cells, and this will be highly dependent on the particular treatment regimen that any individual patient has received. Chemotherapy, radiotherapy, and thymic aging are all associated with decreasing numbers of naive T cells.40
It might be argued that the purification of naive cells before transduction is not necessary, because most of the cells at the end of a stimulation of whole PBMCs could be of naive origin due to their relatively high initial frequency and superior expansion. However, it may still be advantageous to initially isolate naive cells for several reasons. First, T cells from less functional memory subsets compete with more functional T cells from the naive subset for the limited space on a tissue culture plate during transduction. In other words, T-cell gene engineering is a “zero-sum game” in which the number of cells that can be transduced, expanded, and used for treatment is finite; when more memory cells are transduced, fewer naive cells can be transduced. Second, adoptive transfer of exhausted effector cells derived from TEM and TEMRA could act as cytokine sinks and detract from the therapy.42 Finally, naive T cells might receive activation and differentiation signals from activated effector populations, driving them into a program of terminal differentiation. For example, CD70 expressed by activated memory cells might trigger its cognate ligand, CD27, on nearby naive cells triggering “infectious activation” of the latter population.43 These factors may in part be responsible for why bulk transduced cells do not generally express highly levels of CCR7.44 Less activated naive populations might also benefit from growth in small molecules that can act as cell-intrinsic modulators of memory formation(CIMMs) for the pharmacologic induction of immunologic memory.45 Thus, isolation of the naive population might improve both the quantity and the quality of infused cells.
The therapeutic potential of naive T cells is increasingly recognized. T cells for adoptive immunotherapy targeting cytomegalovirus, adenovirus, and Epstein Barr virus can be generated from the naive cells of cord blood.46 Naive cord blood T cells have also been expanded and genetically modified to express a chimeric antigen receptor for treatment of CD19+ B-cell acute lymphoblastic leukemia.47 Thus, work from diverse sources indicates the possible utility of the naive T-cell subset.
Work with CD4+ T-cell populations also indicates the enhanced antitumor potency of cells derived from the naive subset. Recently published experiments have revealed the ability of adoptively transferred naive CD4+ T cells to differentiate into effector cells in vivo and eradicate tumors.48 In the allogeneic transplant setting, naive CD4+ T cells proliferate and persist better than memory subsets, and cause greater graft-versus-host disease than memory subsets,49 although the mechanisms underlying these observations remain a matter of considerable discussion. A recent study from Marrack's group concluded that robust memory T-cell responses are due to increased precursor frequencies and not to any enhanced replicative potential of memory cells, whose proliferation is limited by the cytokines that they produce.50 Thus, there is emerging appreciation for the value of naive cells in adoptive immunotherapy. The data presented herein support further study of naive cells as a source of effector cells for patient treatment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Megan Bachinski for editorial assistance and Arnold Mixon and Shawn Farid for help with cytofluorometry.
National Institutes of Health
Authorship
Contribution: C.S.H. and Z.A.B. designed and performed the experiments, analyzed the data, created the figures, and wrote the manuscript; L.G., Z.Y., W.R.B., J.H., C.A.K., L.A.J., S.P.K., S.Y., P.M., D.C.P., C.D.S, R.A.M., and P.F.R. performed experiments and edited the manuscript; and S.A.R. and N.P.R. directed the project, designed experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicholas P. Restifo, NCI, NIH, Clinical Research Center, Rm 3-5816, 10 Center Dr, Bethesda, MD 20892; e-mail: restifo@nih.gov.
References
Author notes
C.S.H. and Z.A.B. contributed equally to this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal