Abstract
In a phase 1/2 two-arm trial, 54 patients with myeloma received autografts followed by ex vivo anti-CD3/anti-CD28 costimulated autologous T cells at day 2 after transplantation. Study patients positive for human leukocyte antigen A2 (arm A, n = 28) also received pneumococcal conjugate vaccine immunizations before and after transplantation and a multipeptide tumor antigen vaccine derived from the human telomerase reverse transcriptase and the antiapoptotic protein survivin. Patients negative for human leukocyte antigen A2 (arm B, n = 26) received the pneumococcal conjugate vaccine only. Patients exhibited robust T-cell recoveries by day 14 with supraphysiologic T-cell counts accompanied by a sustained reduction in regulatory T cells. The median event-free survival (EFS) for all patients is 20 months (95% confidence interval, 14.6-24.7 months); the projected 3-year overall survival is 83%. A subset of patients in arm A (36%) developed immune responses to the tumor antigen vaccine by tetramer assays, but this cohort did not exhibit better EFS. Higher posttransplantation CD4+ T-cell counts and a lower percentage of FOXP3+ T cells were associated with improved EFS. Patients exhibited accelerated polyclonal immunoglobulin recovery compared with patients without T-cell transfers. Adoptive transfer of tumor antigen vaccine-primed and costimulated T cells leads to augmented and accelerated cellular and humoral immune reconstitution, including antitumor immunity, after autologous stem cell transplantation for myeloma. This study was registered at www.clinicaltrials.gov as NCT00499577.
Introduction
Autologous stem cell transplantations (ASCTs) for myeloma leads to complete responses and extended event-free survival (EFS) in ∼ 20%-40% of patients.1-3 However, even after tandem transplantations, the 10-year EFS is < 20%, and the frequency of cure is < 10%.4 Allogeneic stem cell transplantations may increase the cure rate through a T-cell-mediated graft-versus-tumor effect, but at the expense of increased treatment-related morbidity and mortality from graft-versus-host disease (GVHD) and infection.5-8 Thus, novel strategies are needed to augment the efficacy of ASCT for myeloma and other hematologic malignancies. Efforts to improve the results of autotransplantation for myeloma and other hematologic malignancies include the use of posttransplantation consolidation chemotherapy or maintenance therapy based on targeted agents such as thalidomide and lenalidomide.9-12 These agents may increase the level of response and the time to progression, but their effect on long-term survival and cure is unknown. Higher lymphocyte counts may predict better disease-free and overall survival (OS) for myeloma both early after autotransplantation and at diagnosis.13,14 Similar associations between higher lymphocyte counts and improved outcome have also been reported for lymphoma and myelodysplastic syndromes.15-18 Furthermore, the absolute lymphocyte count at the time of first relapse from large cell lymphoma predicted subsequent progression-free and OS.19
Our objective has been to develop a strategy for inducing an effective antitumor immune response during the posttransplantation period to control or eliminate residual disease. In theory, the posttransplantation phase should be highly amenable to the application of immunotherapy because of a lower tumor burden. However, after high-dose therapy, the immune system is characterized by immune cell depletion and impaired function that may last for years.20,21
We hypothesized that enforced T-cell recovery by adoptive transfer of ex vivo costimulated autologous T cells might improve EFS or OS after autotransplantation for hematologic neoplasms through augmentation or restoration of host antitumor immunity. In addition, enhanced numeric and functional recovery of T cells might provide a platform for posttransplantation tumor vaccine immunotherapy. In our studies, ex vivo costimulation involved coculture of autologous T cells with paramagnetic beads that deliver CD3 and CD28 signals designed to reverse T-cell anergy.22-26 On the basis of this hypothesis, a randomized clinical trial was performed in which 54 patients with myeloma received costimulated autologous T cells after autotransplantation, along with immunizations with a 7-valent pneumococcal conjugate vaccine (PCV; Prevnar; Wyeth).27 One of the key observations from this earlier study was that transfers of ∼ 1010 ex vivo costimulated autologous T cells on day 12 after transplantation led to significantly higher CD4 and CD8 T-cell counts at day 42 after transplantation. In addition, combined T-cell/vaccine immunotherapy could induce vaccine-specific T-cell and antibody immune responses early after transplantation, especially when patients were immunized before T-cell collection and ex vivo expansion. The latter principle was recently reinforced by a parallel randomized study that showed that seroconversion to an influenza vaccine required pretransplantation in vivo priming of autologous T cells before collection, expansion, and adoptive transfer.28
With the use of the strategy of combining immunizations before and after transplantation with early infusions of vaccine-primed and ex vivo costimulated T cells, a new trial was developed with 2 main objectives: (1) to investigate the clinical effects of transferring T cells at day 2 after transplantation, which is 10 days earlier than in our previous study; and (2) to investigate whether the combination strategy could generate immune responses to a myeloma tumor antigen vaccine. The rationale for infusing cells at day 2 was to further exploit the stimulatory cytokine milieu induced by severe lymphopenia (eg, free interleukin-15 [IL-15], IL-7) that may drive homeostatic lymphocyte expansion. In addition, earlier and more robust T-cell recovery might help to promote immune responses to a tumor antigen/self-antigen vaccine, a significantly more challenging task than generating immune responses to a microbial vaccine. In the present study, 54 adults who received autografts for myeloma received ≤ 5 × 1010 (∼ 109/kg) T cells at day 2, along with immunizations before and after transplantation with PCV. In addition, patients who were positive for human leukocyte antigen A2 (HLA-A2) received a multipeptide tumor antigen vaccine that was based on peptides derived from human telomerase reverse transcriptase (hTERT) and survivin, 2 “universal” tumor antigens that are often overexpressed in myeloma and may have prognostic relevance.29-32 Naturally occurring CD8+ T cells recognizing epitopes from these antigens have been described in patients with myeloma.33,34
In our initial description of the trial of T-cell transfer on day 2 after transplantation, we reported that the T-cell infusion induced robust lymphocyte recovery after autotransplantation with median CD3, CD4, and CD8 counts at day 14 after transplantation of 4198, 1545, and 2858 cells/μL, respectively.35 In addition, 16% of patients developed a T-cell “engraftment syndrome” with features of grade 1-3 GVHD. These lymphocyte levels were dramatically higher than were observed in our first trial of transfers on day 12 after transplantation, and the occurrence of clinically significant auto-GVHD was also new, suggesting an important schedule-dependent effect of early T-cell infusions on immune recovery. Here, we report the clinical outcomes of the 54 patients in this posttransplantation immunotherapy trial as well as the immune responses to the hTERT/survivin multipeptide tumor antigen vaccine. By analysis of the biologic correlates of immune function, we also identified predictive biomarkers of EFS.
Methods
Patients
Study participants were ≥ 18 years old with symptomatic multiple myeloma. Patients received first-line therapy with ≥ 3 cycles of standard regimens (typically bortezomib, thalidomide or lenalidomide plus dexamethasone) by their referring oncologist. For enrollment, patients were required to have measurable disease (based on serum/urine electrophoresis studies or serum-free light chain studies); patients in complete remission were not eligible unless they had high-risk cytogenetic features (eg, chromosome 13 or 17 deletions, 4;14 or 14;16 translocations, or complex karyotypes). All patients had adequate organ function as defined by serum creatinine levels < 3.0 mg/dL, left ventricular ejection fraction > 45%, and lung function parameters > 40% predicted. All participants gave written informed consent in accordance with the Declaration of Helsinki; study approval was obtained from the Institutional Review Boards of the University of Maryland and the University of Pennsylvania and the Food and Drug Administration.
Trial design
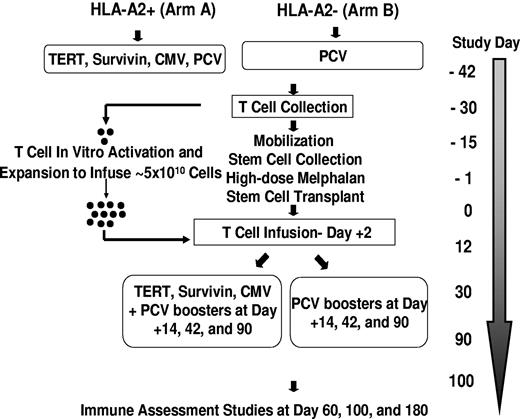
The design of the trial is depicted in Figure 1. Briefly, patients were first tested for HLA-A2 status: patients positive for HLA-A2 (including any A2 allele) were assigned to arm A and patients negative for HLA-A2 were assigned to arm B. Patients who were A2 positive (arm A) were immunized with 100 μg of each of the following peptides: (1) hTERT I540 peptide (ILAKFLHWL),36 (2) hTERT R572Y peptide (YLFFYRKSV),37 (3) hTERT D988Y peptide (YLQVNSLQTV),37 (4) survivin Sur1M2 peptide (LMLGEFLKL),38 and(5) cytomegalovirus control peptide N495 (NLVPMVATV).39 Immunizations consisted of aqueous solutions of peptide (each peptide > 92% pure and good manufacturing grade; Merck Biosciences AG) emulsified in the adjuvant Montanide ISA 51 (Seppic Inc) and delivered subcutaneously in the thigh (right thigh, hTERT I540, hTERT R572Y, and hTERT D988Y peptide emulsion; left thigh, Sur1M2 and cytomegalovirus N495 peptide emulsion). Sargramostim (clinical grade granulocyte-macrophage colony-stimulating factor; Berlex Laboratories, Inc) was also given subcutaneously at each of the 2 peptide injection sites (70 μg per vaccination). Patients in arm A also received an intramuscular injection of the PCV into the nondominant deltoid. Patients negative for HLA-A2 (arm B) received the PCV immunization only along with 1 injection of granulocyte-macrophage colony-stimulating factor (70 μg) into each thigh.
Approximately 10 days after the first set of immunizations all patients had steady-state apheresis to collect ∼ 1 × 108 mononuclear cells per kilogram body weight. Patients then proceeded to stem cell mobilization with the use of one of several regimens (most commonly cyclophosphamide at a dose of 1.5-4.5 g/m2) followed by subcutaneous injections of granulocyte colony-stimulating factor (10 μg/kg). High-dose therapy was melphalan (200 mg/m2) followed by infusions of autologous stem cells (> 2 × 106 CD34+ cells/kg of body weight) at day 0. Costimulated autologous T cells were infused on day 2. Supportive care measures included antibiotic prophylaxis and administration of granulocyte colony-stimulating factor, starting on day 5. Three additional sets of immunizations were given at days 14, 42, and 90 after transplantation with the use of the same arm-specific vaccine composition and procedures that were used for the first immunization.
T-cell expansion and adoptive transfer
The mononuclear cell apheresis product was monocyte-depleted by counter flow centrifugal elutriation (CaridianBCT Elutra; Cell Separation System) because monocytes may inhibit lymphocyte proliferation. Monocyte-depleted mononuclear cells were cryopreserved until 9-12 days before the scheduled reinfusion date (day 2 after transplantation). Cells were thawed and cocultured with Dynal paramagnetic M-450 beads (Dynal Invitrogen) coated with anti-CD3 (OKT3; Ortho Biotech)/anti-CD28 (clone 9.3) at a ratio of 3 beads per cell first in a Baxter Lifecell flask and subsequently in the WAVE bioreactor system (GE Healthcare Biosciences).40 Additional details of T-cell expansion and harvesting are described elsewhere35 and in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The harvested cells were transported by courier from the cell production facility to the patient and infused on the same day (day 2 of transplantation). The cells were infused > 20 to 60 minutes without a leukocyte filter, after premedication with acetaminophen and diphenhydramine. The target number of costimulated T cells for infusion was ∼ 5 × 1010, which was 5-fold greater than that used in our previous trial. Of the 54 expansions, one product failed to meet release criteria because of bacterial contamination; after a second expansion, cells were successfully infused at day 16.
Immunoassays and phenotyping
In vitro stimulation.
In vitro peptide stimulation of peripheral blood mononuclear cells to assess immune response was performed as previously described.41 Peptide/major histocompatibility complex class I tetramer analysis was performed with soluble peptide/HLA-A2 tetramers purchased from Beckman Coulter Immunomics, as previously described.41
Proliferation assays.
T-cell responses to the CRM197 and hTERT/survivin peptides (Merck Biosciences AG) were measured by CFSE (5,6-carboxyfluorescein diacetate, succinimidyl ester) staining of responder T cells as previously described.27 Briefly, after 6 days in culture with media alone (negative control), staphylococcal enterotoxin B (positive control; 100 ng/mL; EMD Chemicals), CRM197 (20 μg/mL; List Biological Laboratories), hTERT/survivin peptide mix (5 μg/mL), or irrelevant peptide (Tax, 5μg/mL), the percentage of CFSEdim cells after gating on live CD8+ or CD4+ T cells was measured by flow cytometry.
Flow cytometric reagents and other materials.
Fluorochrome-conjugated monoclonal antibody used were as follows: phycoerythrin (PE)–indocyanine 7–CD3 clone SK7, allophycocyanin (APC)–C7-CD4 clone RPA-T4, peridinin chlorophyll protein complex–CD4 clone SK3, APC–indocyanine 7–CD8 clone SK1, peridinin chlorophyll protein complex–CD14 clone MϕP9, fluorescein isothiocyanate (FITC)–CD16 clone 3G8, APC-CD19 clone HIB19, APC-CD25 clone MA-251, FITC-CD27 clone M-T271, APC-CD28 clone CD28.2, APC-CD45RA clone HI100, FITC-CD45RO clone UCHL1, PE-CD56 clone B159, APC-CD69 clone FN50, PE-CD127 clone hIL-7R-M21, PE-HLA-DR clone L243, PE-CCR7 clone 3D12 (BD Biosciences); APC-CD8 (clone B9.11) (Beckman Coulter); Alexa Fluor 488–OXP3 clone 259D (BioLegend). Flow cytometry was performed with a custom FACSCanto cytometer and FACSDiva software (BD Biosciences Immunocytochemistry Systems). Data were analyzed with FlowJo software (TreeStar Inc). Intracellular staining for FOXP3 was performed with a fixation/permeabilization kit (eBioscience).
Antibody responses to the PCV were assessed by enzyme-linked immunoabsorbent assay binding assays for serotypes 6B, 14, 19F, 23F as previously described.27 Titers were reported in μg/mL with the use of the reference standard serum 89SF.
Statistical methods
The chi-square test (for categorical variables) and Wilcoxon test (for continuous variables) were used to compare patient characteristics between the 2 study arms. Pearson correlation coefficient was used for correlation analyses. Linear mixed models were used to analyze the effects of infused T-cell number on posttransplantation T-cell recovery. EFS and OS were calculated from the date of transplantation (day 0) according to the Kaplan-Meier product limit method. The log-rank test and Cox proportional hazards models were used to analyze the relationships of patient characteristics and immune parameters with EFS. The observation times were day 14, day 60, day 100, and day 180 as specified per protocol. Because the observation times were slightly different between the current trial and previous trials and some observations were missing, to compare patient immune responses between the trials, the generalized t test was used on the basis of the expectation-maximization algorithm.42 A P value < .05 was used for statistical significance.
Results
Patient characteristics
From December 2006 to February 2009, we enrolled 56 patients at the 2 participating institutions. After the initial priming immunization, 2 patients (both arm A) did not mobilize adequate numbers of stem cells for transplantation and did not remain in the study. Table 1 shows the main clinical characteristics for the 54 patients (arm A = 28; arm B = 26) who proceeded to transplantation (see supplemental Table 1 for more details). The mean age of study participants was 55 years with 52% men and 39% African Americans. This cohort of higher risk patients had a mean marrow plasmacytosis of 27% (range, 1%-95%) at enrollment despite extensive prior treatment with thalidomide and lenalidomide or bortezomib or both, and 39% had abnormal cytogenetic studies (of which 15 of 21 or 71% had complex or other high-risk abnormalities). In addition, 50% of patients had ≥ 2 courses of prior therapy. The 2 arms were well balanced except that 18% of patient in arm A were African American compared with 62% of patients in arm B (P = .003), reflecting the known lower frequency of HLA-A2 alleles in African Americans compared with other ethnicities.
Characteristics of patients in arm A and arm B
| . | Values . | Arm A . | Arm B . | P . |
|---|---|---|---|---|
| No. of patients | 54 | 28 | 26 | |
| Age, y, range (mean) | 37-68 (55) | 45-68 (55.6) | 37-67 (54.3) | .74 |
| Race | ||||
| White, n (%) | 32 (59) | 22 (78) | 10 (38) | .003* |
| African American, n (%) | 21(39) | 5 (18) | 16 (62) | |
| Asian, n (%) | 1 (2) | 1 (4) | 0 | |
| Sex | ||||
| Male, n (%) | 28 (52) | 16 (57) | 12 (46) | .59 |
| Female, n (%) | 26 (48) | 12 (43) | 14 (54) | |
| Myeloma subtypes | ||||
| IgA, n | 15 | 9 | 6 | .08 |
| IgG, n | 35 | 15 | 20 | |
| Light chains, n | 4 | 4 | 0 | |
| Thal maint (patients EFS > 180days) | ||||
| Yes, n | 24 | 7 | 17 | .003* |
| No, n | 22 | 17 | 5 | |
| β-2 microglobulin levels at EN, range (mean) | 0.87-4.13 (1.91) | 0.87-3.33 (1.70) | 0.93-4.13 (2.11) | .03* |
| . | Values . | Arm A . | Arm B . | P . |
|---|---|---|---|---|
| No. of patients | 54 | 28 | 26 | |
| Age, y, range (mean) | 37-68 (55) | 45-68 (55.6) | 37-67 (54.3) | .74 |
| Race | ||||
| White, n (%) | 32 (59) | 22 (78) | 10 (38) | .003* |
| African American, n (%) | 21(39) | 5 (18) | 16 (62) | |
| Asian, n (%) | 1 (2) | 1 (4) | 0 | |
| Sex | ||||
| Male, n (%) | 28 (52) | 16 (57) | 12 (46) | .59 |
| Female, n (%) | 26 (48) | 12 (43) | 14 (54) | |
| Myeloma subtypes | ||||
| IgA, n | 15 | 9 | 6 | .08 |
| IgG, n | 35 | 15 | 20 | |
| Light chains, n | 4 | 4 | 0 | |
| Thal maint (patients EFS > 180days) | ||||
| Yes, n | 24 | 7 | 17 | .003* |
| No, n | 22 | 17 | 5 | |
| β-2 microglobulin levels at EN, range (mean) | 0.87-4.13 (1.91) | 0.87-3.33 (1.70) | 0.93-4.13 (2.11) | .03* |
Thal maint indicates thalidomide maintenance for patients who remained event-free at 180 days after transplantation; and EN, enrollment.
Statistically significant.
Toxicities from T-cell infusions and immunizations
Table 2 shows the early and late toxicities that were possibly, probably, or definitely related to the T-cell transfers. The most common early effects were mild-to-moderate chills and rigors (57% of all patients), low-grade fevers, and mild nausea/vomiting. Arms A and B had similar patterns of early infusion-related toxicities except for an excess of mild nausea/vomiting for patients in arm B (P = .02). Late effects of T-cell transfers included grade I/II rashes in > 85% of the entire cohort of patients. These rashes primarily developed on the face, neck, upper chest, and upper back. Late effects after T-cell infusions were also similar between arms A and B, except for a significant excess of diarrhea (P = .001) and anorexia/nausea (P = .04) in arm A. Table 3 depicts the injection site and constitutional reactions after hTERT/survivin multipeptide vaccine that were reported by patients in arm A in self-assessment symptom diaries. All reactions were mild or moderate in degree, with injection site pain, induration/redness, loss of energy, and myalgias being the most common. There were no discernible trends in reactivity patterns over time, except for a significant increase after transplantation in the frequency of injection site induration or redness zones of > 50 mm, probably because of development of delayed-type hypersensitivity (DTH) reactions in a proportion of vaccinated patients. In patient MD-012, the day-90 immunization with the hTERT/survivin multipeptide vaccine elicited a 50-mm zone of redness and induration at the previous day-42 injection site.
T cell–related toxicities (arm A vs arm B)
| . | Grade I, n . | Grade II, n . | Grade III, n . | Totals, n (%) . | P . | ||||
|---|---|---|---|---|---|---|---|---|---|
| A . | B . | A . | B . | A . | B . | A . | B . | ||
| Event (0-48 h) | |||||||||
| Rigors/chills | 7 | 6 | 11 | 7 | 0 | 0 | 18 (64) | 13 (50) | NS |
| Nausea/vomiting | 1 | 7 | 0 | 0 | 0 | 0 | 1 (4) | 7 (27) | .02* |
| Fever | 3 | 4 | 0 | 0 | 0 | 0 | 3 (11) | 4 (15) | NS |
| Headache/pain | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 (12) | NS |
| Event (onset > 48 h) | |||||||||
| Rash | 19 | 19 | 4 | 4 | 0 | 0 | 23 (82) | 23 (88) | NS |
| Fever | 6 | 4 | 1 | 0 | 1 | 0 | 8 (29) | 4 (15) | NS |
| Gut GVHD | 1 | 0 | 3 | 2 | 0 | 1 | 4 (14) | 3 (12) | NS |
| Arthralgias | 3 | 4 | 0 | 1 | 0 | 0 | 3 (11) | 5 (19) | NS |
| Myalgias | 5 | 0 | 0 | 0 | 0 | 0 | 5 (18) | 0 | .05 |
| Headache/pain | 4 | 1 | 3 | 0 | 0 | 0 | 7 (25) | 1 (4) | .05 |
| Anorexia/nausea | 6 | 2 | 2 | 0 | 1 | 0 | 9 (32) | 2 (8) | .04* |
| Conjunctivitis | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 (12) | NS |
| Fatigue | 6 | 0 | 1 | 1 | 0 | 0 | 7 (25) | 1 (4) | .05 |
| Diarrhea | 10 | 3 | 6 | 1 | 2 | 1 | 18 (64) | 5 (19) | .001* |
| . | Grade I, n . | Grade II, n . | Grade III, n . | Totals, n (%) . | P . | ||||
|---|---|---|---|---|---|---|---|---|---|
| A . | B . | A . | B . | A . | B . | A . | B . | ||
| Event (0-48 h) | |||||||||
| Rigors/chills | 7 | 6 | 11 | 7 | 0 | 0 | 18 (64) | 13 (50) | NS |
| Nausea/vomiting | 1 | 7 | 0 | 0 | 0 | 0 | 1 (4) | 7 (27) | .02* |
| Fever | 3 | 4 | 0 | 0 | 0 | 0 | 3 (11) | 4 (15) | NS |
| Headache/pain | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 (12) | NS |
| Event (onset > 48 h) | |||||||||
| Rash | 19 | 19 | 4 | 4 | 0 | 0 | 23 (82) | 23 (88) | NS |
| Fever | 6 | 4 | 1 | 0 | 1 | 0 | 8 (29) | 4 (15) | NS |
| Gut GVHD | 1 | 0 | 3 | 2 | 0 | 1 | 4 (14) | 3 (12) | NS |
| Arthralgias | 3 | 4 | 0 | 1 | 0 | 0 | 3 (11) | 5 (19) | NS |
| Myalgias | 5 | 0 | 0 | 0 | 0 | 0 | 5 (18) | 0 | .05 |
| Headache/pain | 4 | 1 | 3 | 0 | 0 | 0 | 7 (25) | 1 (4) | .05 |
| Anorexia/nausea | 6 | 2 | 2 | 0 | 1 | 0 | 9 (32) | 2 (8) | .04* |
| Conjunctivitis | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 (12) | NS |
| Fatigue | 6 | 0 | 1 | 1 | 0 | 0 | 7 (25) | 1 (4) | .05 |
| Diarrhea | 10 | 3 | 6 | 1 | 2 | 1 | 18 (64) | 5 (19) | .001* |
Shown are toxicities which were possibly, probably, or definitely related to adoptive T-cell transfers either early (0-48 hours) after transfer or late (> 48 hours) after transfer. Severity grades of I, II and III are shown; no grade IV or V toxicities were observed. Other toxicities (not listed), such as hypertension, hypoxia/pulmonary, rash, and diarrhea (0-48 hours); cytopenias, neuropathy, hepatic(elevated aspartate aminotransferase/alanine aminotransferase), eosinophilia, renal/edema, mucositis, and mental status changes (> 48 hours) occurred in individual patients in both groups with no significant differences between the 2 groups.
GVHD indicates graft-versus-host disease.
Significant differences between total number of events for arms A and B for each type of toxicity (NS indicates P > .05).
Reactions to hTERT/survivin vaccinations in patients in arm A (n = 28) at different time points
| . | Before transplantation, n (%) . | Day 14, n (%) . | Day 42, n (%) . | Day 90, n (%) . |
|---|---|---|---|---|
| Temperature ≥ 100°F | 3 (11) | 9 (32)* | 2 (7) | 1 (4) |
| Pain at injection site(≥ grade II) | 9 (32) | 9 (32) | 18 (64) | 17 (61) |
| Loss of appetite (≥ grade II) | 2 (7) | 8 (29) | 6 (21) | 3 (11) |
| Loss of energy (≥ grade II) | 6 (21) | 13 (46) | 12 (43) | 10 (36) |
| Headaches (≥ grade II) | 2 (7) | 2 (7) | 3 (11) | 4 (14) |
| Muscle aches (≥ grade II) | 9 (32) | 8 (29) | 12 (43) | 14 (50) |
| Nausea/vomiting (≥ grade II) | 1 (4) | 10 (36)* | 5 (18) | 1 (4) |
| Redness or induration(≥ 12 mm) | 13 (46) | 10 (36) | 20 (71)* | 17 (61) |
| Redness or induration(≥ 36 mm) | 11 (39) | 7 (25) | 13 (46) | 16 (57) |
| Redness or induration(> 50 mm) | 7 (25) | 2 (7) | 6 (21) | 12 (43)* |
| . | Before transplantation, n (%) . | Day 14, n (%) . | Day 42, n (%) . | Day 90, n (%) . |
|---|---|---|---|---|
| Temperature ≥ 100°F | 3 (11) | 9 (32)* | 2 (7) | 1 (4) |
| Pain at injection site(≥ grade II) | 9 (32) | 9 (32) | 18 (64) | 17 (61) |
| Loss of appetite (≥ grade II) | 2 (7) | 8 (29) | 6 (21) | 3 (11) |
| Loss of energy (≥ grade II) | 6 (21) | 13 (46) | 12 (43) | 10 (36) |
| Headaches (≥ grade II) | 2 (7) | 2 (7) | 3 (11) | 4 (14) |
| Muscle aches (≥ grade II) | 9 (32) | 8 (29) | 12 (43) | 14 (50) |
| Nausea/vomiting (≥ grade II) | 1 (4) | 10 (36)* | 5 (18) | 1 (4) |
| Redness or induration(≥ 12 mm) | 13 (46) | 10 (36) | 20 (71)* | 17 (61) |
| Redness or induration(≥ 36 mm) | 11 (39) | 7 (25) | 13 (46) | 16 (57) |
| Redness or induration(> 50 mm) | 7 (25) | 2 (7) | 6 (21) | 12 (43)* |
Significantly higher than some of the earlier time points (P < .05), except for temperature > 100°F, which was significantly higher than a later time point, and nausea/vomiting, which was significantly higher than a later time point and an earlier time point.
EFS and OS
The 3-year projected OS for the entire cohort is 83% with no difference between the 2 arms (Figure 2A). A total of 7 patients have died, all of relapse of myeloma: 4 in arm A and 3 in arm B. Clinical responses (defined as partial response, very good partial response, nodular complete response, and complete response) at days 100 (n = 40) and 180 (n = 33) were predictive of better EFS (P = .005 and P < .0001, respectively). Figure 2B shows the Kaplan-Meier EFS for the entire cohort of 54 patients (black line) as well as the EFS curves for arm A (red line) and arm B (blue line). The median EFS for the entire cohort is 20 months (95% confidence interval, 14.6-24.7 months). The EFS for patients in arm A and arm B are significantly different (P = .0068). The 2-year projected EFS is ∼ 65% for patients in arm B (no hTERT/survivin vaccine) and 25% for patients in arm A. The main contributor to this difference is probably the differential usage of thalidomide maintenance between the 2 arms, which was an optional intervention specified to begin on day 180 per protocol. Among the 46 patients whose EFS exceeded 180 days when the thalidomide maintenance could be started, 17 of 22 patients in arm B received thalidomide compared with only 7 of 24 patients in arm A (P = .003). For the cohort of patients who remained event free at 180 days after transplantation, Figure 2C shows the difference in EFS between patients who received thalidomide maintenance (blue line) and patients who did not (red line) (P = .0089). After adjustment for the effect of thalidomide maintenance with the use of a stratified log-rank test, the P value for the difference in EFS between arms A and B becomes nonsignificant (P = .21).
Kaplan-Meier survival curves. (A) Overall survival. (B) Event-free survivals (EFS) according to assignment to study arm. EFS for arm B is superior to EFS for arm A (P = .0068). (C) EFS for patients who remained event-free at day 180 after transplantation and received thalidomide maintenance had improved EFS compared with patients who did not receive thalidomide (P = .0089).
Kaplan-Meier survival curves. (A) Overall survival. (B) Event-free survivals (EFS) according to assignment to study arm. EFS for arm B is superior to EFS for arm A (P = .0068). (C) EFS for patients who remained event-free at day 180 after transplantation and received thalidomide maintenance had improved EFS compared with patients who did not receive thalidomide (P = .0089).
Immune responses to the hTERT/survivin tumor antigen vaccine
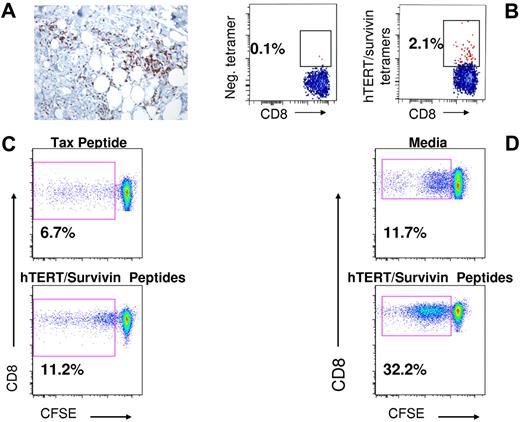
A main objective of the study was to determine whether adoptive transfer of vaccine-primed autologous T cells could elicit early immune responses to the hTERT/survivin multipeptide vaccine. T-cell responses to the hTERT/survivin tumor antigens were assessed by tetramer analysis at serial time points after in vitro stimulation of mononuclear cells. The chief immunologic endpoint was the frequency of positive tetramer responses, which was defined as both a staining level of > 0.1% and a > 3-fold increase versus the enrollment/baseline time point. Figure 3A shows the tetramer responses for the 10 patients (36%) who exhibited positive responses with the use of a scale of 0%-1%, whereas Figure 3B shows the full spectrum of responses. Figure 4A is a deep skin biopsy of the DTH lesion from UMD-012 showing a perivascular CD3+/CD8+ T-cell infiltrate in the subcutaneous fat that also contained hTERT/survivin-specific CD8+ T cells by tetramer analysis (Figure 4B). Figure 4C and D shows the proliferative responses by CFSE dilution for one representative hTERT/survivin tetramer responder at day 180 after transplantation and a second patient at day 14 after transplantation. Both patients have substantial increases in CFSEdim cells after stimulation with the hTERT/survivin peptide mix, consistent with the significant frequency of tetramer-reactive cells shown in Figure 3. However, we also observed an increased background proliferation in the cultures after incubation of the peripheral blood mononuclear cells with the irrelevant Tax peptide as well as in cultures of tissue culture media only.
Tetramer immune responses to hTERT/survivin vaccine. (A) Bar graph showing 10 patients (of 28; 36%) with positive tetramer responses defined as > 3-fold increase in tetramer staining compared with enrollment/baseline and minimum level of 0.1%. (B) Same patients depicted in panel A but using an expanded y-axis to show the full spectrum of responses.
Tetramer immune responses to hTERT/survivin vaccine. (A) Bar graph showing 10 patients (of 28; 36%) with positive tetramer responses defined as > 3-fold increase in tetramer staining compared with enrollment/baseline and minimum level of 0.1%. (B) Same patients depicted in panel A but using an expanded y-axis to show the full spectrum of responses.
Functional immune responses in specific patients. (A) Deep skin biopsy of ≥ 5-cm area of induration (from patient MD012) showing CD8+/CD3+ T-cell infiltrate. (B) Tetramer analysis of T cells extracted from the skin biopsy depicted in panel A; 2.1% of the cells analyzed exhibited hTERT/survivin tetramer staining versus 0.1% in the control (tetramer negative) sample. (C) Proliferative response of CD8+ T cells (from patient UPCC/13406-11) after stimulation with hTERT/survivin peptide mix by CFSE dilution analysis; percentage of CFSEdim was 11.2% in the hTERT/survivin-stimulated cells (bottom) versus 6.67% in cells stimulated with an irrelevant peptide derived from the Tax protein (top) and 6.2% in cells exposed to medium only (data not shown). (D) Proliferative response of CD8+ T cells from a second immunized patient (UPCC/13406-22) after stimulation with hTERT/survivin peptide mix by CFSE dilution analysis; percentage of CFSEdim was 33.2% in the hTERT/survivin-stimulated cells (bottom) versus 11.7% in cells that were incubated in media only without peptide stimulation (top).
Functional immune responses in specific patients. (A) Deep skin biopsy of ≥ 5-cm area of induration (from patient MD012) showing CD8+/CD3+ T-cell infiltrate. (B) Tetramer analysis of T cells extracted from the skin biopsy depicted in panel A; 2.1% of the cells analyzed exhibited hTERT/survivin tetramer staining versus 0.1% in the control (tetramer negative) sample. (C) Proliferative response of CD8+ T cells (from patient UPCC/13406-11) after stimulation with hTERT/survivin peptide mix by CFSE dilution analysis; percentage of CFSEdim was 11.2% in the hTERT/survivin-stimulated cells (bottom) versus 6.67% in cells stimulated with an irrelevant peptide derived from the Tax protein (top) and 6.2% in cells exposed to medium only (data not shown). (D) Proliferative response of CD8+ T cells from a second immunized patient (UPCC/13406-22) after stimulation with hTERT/survivin peptide mix by CFSE dilution analysis; percentage of CFSEdim was 33.2% in the hTERT/survivin-stimulated cells (bottom) versus 11.7% in cells that were incubated in media only without peptide stimulation (top).
Immune responses to the PCV
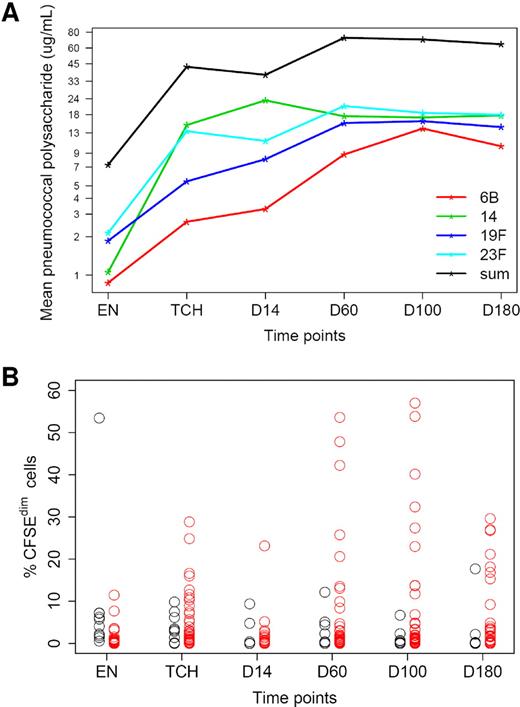
The relatively large number of patients (n = 54) who received the combination of PCV-primed autologous T cells by early adoptive transfer followed by booster immunizations with the PCV provided the opportunity to evaluate more accurately the frequency and magnitude of the T-cell and B-cell responses to this microbial conjugate vaccine. Figure 5A shows the mean pneumococcal immunoglobulin G (IgG) levels for each of the 4 serotypes tested (6B, 14, 19F, 23F), as well as the sum of all 4 serotypes at serial time points before and after stem cell transplantation. The mean antibody responses for all 4 serotypes increased progressively after transplantation and far exceed the level of 0.5 μg/mL considered to be “protective” against infection. The percentage of patients who had antibody responses (> 4-fold increase in titers after vaccination compared with baseline) to > 1, > 2, > 3, or 4 of the 7 serotypes carried by the PCV were 94% (n = 50), 75% (n = 40), 58% (n = 31), and 47% (n = 25), respectively. The proliferative T-cell responses to the CRM-197 carrier protein were assayed by CFSE dilution studies (Figure 5B). In Figure 5B, the red circles represent “responders” who had > 3-fold increases in the percentage of CFSEdim cells at ≥ 1 time points after transplantation, whereas the black circles represent “nonresponders.” With the use of > 2, > 3, and > 4-fold increases in the CFSEdim population compared with enrollment/baseline, 86%, 80%, and 73% of the patients had positive T-cell responses to the CRM-197 carrier protein, respectively.
B- and T-cell responses to PCV vaccine. (A) Log-transformed plot of mean serum IgG antibody responses for each of the 4 PCV serotypes tested over the course of the study. (B) CD4+ T-cell responses to the CRM-197 carrier protein on the basis of proliferation assays with the use of CFSE dilution; percentage of CFSEdim cells after CRM-197 stimulation at various time points for 49 total patients are shown. Black circles indicate nonresponders; red circles, responders. A responder is defined as a patient having at least a 3-fold increase of the enrollment measurement at ≥ 1 posttransplantation time points.
B- and T-cell responses to PCV vaccine. (A) Log-transformed plot of mean serum IgG antibody responses for each of the 4 PCV serotypes tested over the course of the study. (B) CD4+ T-cell responses to the CRM-197 carrier protein on the basis of proliferation assays with the use of CFSE dilution; percentage of CFSEdim cells after CRM-197 stimulation at various time points for 49 total patients are shown. Black circles indicate nonresponders; red circles, responders. A responder is defined as a patient having at least a 3-fold increase of the enrollment measurement at ≥ 1 posttransplantation time points.
Immune cell recovery and improved EFS
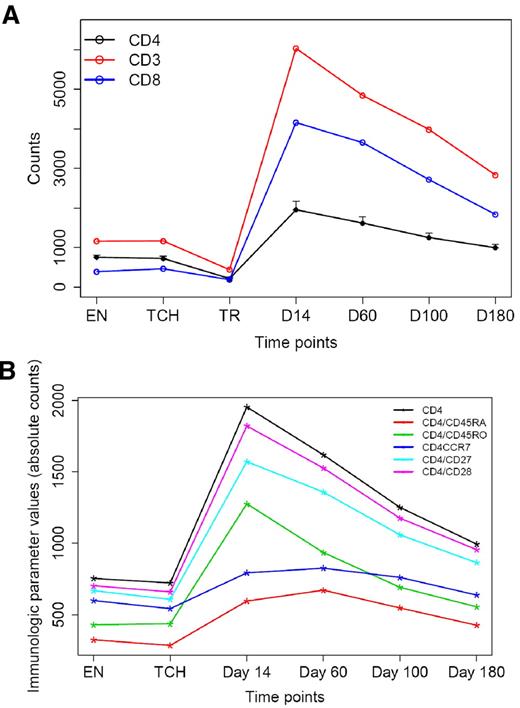
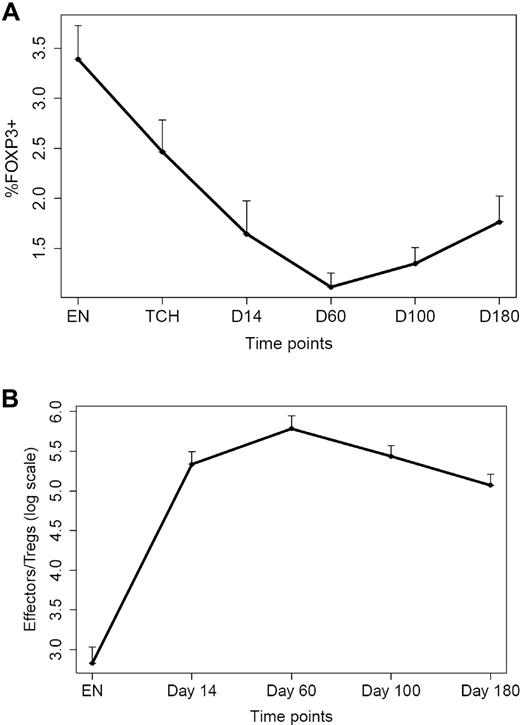
Although disease status before transplantation did not influence T-cell recovery after transplantation, higher infused CD4+ T-cell doses significantly increased CD4 counts after transplantation (slope = 0.66 in log scale; P = .0001) and higher infused CD8+ T-cell doses significantly increased CD8 recovery after transplantation (slope = 0.29; P < .0001). The abundance of longitudinal phenotypic and functional immunologic data that were collected during the course of this study also afforded the opportunity to analyze relationships between parameters of immune recovery and clinical outcome. Because thalidomide maintenance resulted in a significant improvement of EFS as mentioned earlier, Cox proportion hazards models stratifying on thalidomide maintenance were used to study correlations between immune parameters and EFS. On the basis of the univariate analysis, we found that higher absolute values of CD4+ T cells were associated with improved EFS for the entire cohort of patients (P = .037 at day 14, P = .048 at day 60, P = .013 at day 100, and P = .034 at day 180, respectively). Higher percentage of CD4+ T cells were also associated with improved EFS (P = .034 at day 14, P = .017 at day 60, and P = .033 at day 100, respectively). Because the percentage of CD8+ T cells was inversely correlated with the percentage of CD4+ T cells, it was not surprising that lower percentages of CD8+ T cells led to improved EFS (P = .017 at day 14, P = .015 at day 60, and P = .049 at day 100, respectively). Certain CD4 and CD8 T-cell subsets (including day 180 CD8+/CD45RO+ T cells) were correlated to EFS as well (see supplemental Table 2). Furthermore, lower percentages of FOXP3+ T cells (Tregs) were associated with improved EFS for the entire cohort of patients (P = .047 at T-cell harvest, P = .002 at day 14, P = .004 at day 60, P < .001 at day 100 and at day 180, respectively) along with a lower number of Tregs at enrollment (P = .01). The mean levels of these phenotypic variables over time for all patients on study are shown in Figures 6 and 7. The percentage of Tregs was significantly lower (1.1% vs 5.5%; P < .0001) and the loge Teff/Treg was significantly higher (5.92 vs 4.45; P = .002) at day 60 compared with a cohort of unselected patients (n = 9) who received an autograft for myeloma without T-cell transfers.
Immune reconstitution with sustained lymphocytosis of multiple T-cell subsets after transplantation. (A) Mean CD3+, CD4+, and CD8+ counts at various time points before and after transplantation. EN indicates enrollment; TCH, T-cell harvest (apheresis); TR, transplantation date. (B) Mean levels of CD4+ subsets before and after transplantation. Bars denote the standard errors.
Immune reconstitution with sustained lymphocytosis of multiple T-cell subsets after transplantation. (A) Mean CD3+, CD4+, and CD8+ counts at various time points before and after transplantation. EN indicates enrollment; TCH, T-cell harvest (apheresis); TR, transplantation date. (B) Mean levels of CD4+ subsets before and after transplantation. Bars denote the standard errors.
Percentage of CD4+FOXP3+ T cells and Teff/Treg ratio before and after transplantation. (A) Percentage of CD4+FOXP3+ T cells at various time points before and after transplantation. Bars denote 1 standard deviation. (B) Loge of Teff/Treg ratio at various time points before and after transplantation. EN indicates enrollment; TCH, T-cell harvest (apheresis).
Percentage of CD4+FOXP3+ T cells and Teff/Treg ratio before and after transplantation. (A) Percentage of CD4+FOXP3+ T cells at various time points before and after transplantation. Bars denote 1 standard deviation. (B) Loge of Teff/Treg ratio at various time points before and after transplantation. EN indicates enrollment; TCH, T-cell harvest (apheresis).
Accelerated immunoglobulin recovery after T-cell transfers
Posttransplantation immunoglobulin levels at serial time points were compared between the current trial and an historical cohort of 102 patients with myeloma who had standard autografts at the University of Maryland site without T-cell transfers.9 To eliminate the effect of the myeloma paraprotein, we compared IgG recovery for the patients with non-IgG myeloma, IgA recovery for the patients with non-IgA myeloma, and IgM recovery for all patients. Early polyclonal recovery rates were significantly greater for the IgG, IgA, and IgM fractions among the patients who received T-cell transfers on day 2 after transplantation compared with the historical control patients (Table 4). The estimated IgA levels among patients with IgG myeloma who received T-cell transfers on day 2 after transplantation were significantly higher than in the historical control patients at days 60, 100, and 180, respectively (Table 5). For the IgA patients, the estimated polyclonal IgG levels were significantly higher in the current trial at day 180 (810 mg/dL vs 611 mg/dL; P = .048). The estimated IgM levels were also significantly higher at day 180 in the current trial (58 mg/dL vs 39 mg/dL; P = .029).
Effect of adoptive T cells on immunoglobulin recovery
| Ig subtype . | Rate (-exT), mg/dL/d . | Rate (+exT), mg/dL/d . | P . |
|---|---|---|---|
| IgA | 0.62 | 1.33 | .041 |
| IgG | 3.41 | 11.22 | .0006 |
| IgM | -0.03 | 0.31 | .0001 |
| Ig subtype . | Rate (-exT), mg/dL/d . | Rate (+exT), mg/dL/d . | P . |
|---|---|---|---|
| IgA | 0.62 | 1.33 | .041 |
| IgG | 3.41 | 11.22 | .0006 |
| IgM | -0.03 | 0.31 | .0001 |
Estimated rates of initial recovery of polyclonal IgA (in patients with non-IgA myeloma), IgG (in patients with non-IgG myeloma), and IgM for 54 patients in current trial who received expanded T cells (+exT) compared with 102 total patients in historical database who did not receive expanded T cells (-exT). P values were significant for immunoglobulin recovery rates.
IgA recovery of patients with IgG myeloma
| IgA at different time points . | -exT (estimated), mg/dL (n = 64) . | +exT (estimated), mg/dL (n = 36) . | P . |
|---|---|---|---|
| Day 60 | 71.7 | 129.8 | .004 |
| Day 100 | 59.4 | 114.4 | .008 |
| Day 180 | 48.7 | 98.4 | .004 |
| IgA at different time points . | -exT (estimated), mg/dL (n = 64) . | +exT (estimated), mg/dL (n = 36) . | P . |
|---|---|---|---|
| Day 60 | 71.7 | 129.8 | .004 |
| Day 100 | 59.4 | 114.4 | .008 |
| Day 180 | 48.7 | 98.4 | .004 |
Estimated values for polyclonal IgA among 36 patients with evaluable IgG (or light chain only) myeloma in current trial who received expanded T cells (+exT) compared with 64 patients with IgG (or light chain only) myeloma in the historical database who did not receive expanded T cells (-exT) at days 60, 100, and 180 after transplantation. P values were significant at each day analyzed.
Discussion
Our long-term goal is to improve antitumor immunity in patients after high-dose chemotherapy, at a point when tumor burden may be reduced. The objective of this protocol was to build on our previous study27 and to further define the schedule-dependent effects of adoptive cell transfer. A secondary objective was to learn whether combination immunotherapy consisting of vaccine and adoptive cell transfer could induce anti-self immunity, in the setting of the profound immunodeficiency after ASCT.20
In our previous study, we found that T-cell transfer on day 12 after stem cell infusion was significantly more effective than on day 100.27 Our current study has shown that T-cell transfers on day 2 result in a substantially accelerated reconstitution of T-cell immunity, as shown by several quantitative and functional measures. An unexpected finding was that T-cell homeostasis was altered after T-cell infusions on day 2, because a sustained T-cell lymphocytosis was observed in most patients. To our knowledge, this is unprecedented, absent treatment of patients with cytokine infusions.
The lymphocytosis may have beneficial clinical consequences, because higher absolute values of CD4+ T cells were significantly predictive of improved EFS at all time points tested after transplantation as were higher levels of CD8+/CD45RO+ T cells at day 180. Patients with higher percentages of CD4+ T cells and correspondingly lower percentage of CD8+ T cells also exhibited better EFS. In addition to the schedule-dependent lymphocytosis after T-cell transfer on day 2, we recently reported that a subset of patients have an engraftment syndrome resembling autologous GVHD35 that was not observed in our previous trial after T-cell infusions on day 12 or day 100.27
In addition to the induction of lymphocytosis, we found that T-cell transfer day 2 was associated with a reduction in the percentage of Tregs after transplantation compared with the baseline levels in the 54 patients and compared with a cohort of patients with myeloma who received an autograft without T-cell transfers. It should be recognized that FOXP3 expression is not entirely specific for Tregs because it can be transiently expressed in activated T cells. The percentage of FOXP3+ T cells (Tregs) was strongly and inversely associated with improved EFS at all time points tested after transplantation. This finding concurs with previous studies that indicate lower Treg levels and especially increased Teff/Treg ratios are associated with enhanced tumor necrosis in clinical trials of immune modulation.43 Our results indicating that myeloablative chemotherapy combined with immune reconstitution after T-cell transfer are notable because to our knowledge other approaches have not led to a substantial reduction in Tregs in patients with advanced cancer. Uncovering the mechanisms leading to the increased Teff/Treg ratio will require further study and may involve a “purging effect” consequent to the relative depletion of Tregs in the adoptively transferred T cells, as well as host-dependent effects during homeostatic expansion in that Tregs may be at a competitive disadvantage compared with effector T cells.
A main goal of our study was to test whether the combination of tumor vaccination and T-cell infusions on day 2 could induce antitumor immunity. Ten patients or 36% had immune responses to the hTERT/survivin multipeptide vaccine. This frequency exceeds the minimum of 7 responses that represented the immunologic efficacy endpoint for the study and compares favorably to the ∼ 20% frequency of cellular or antibody immune responses to idiotype vaccines that were administered to patients with myeloma after autotransplantation.44,45 A subset of patients had a vigorous response to the vaccine; however, the responses were generally modest compared with the robust cellular and humoral immunities that were nearly universally observed after the PCV. Thus, the immune response frequency to the multipeptide tumor antigen vaccine, although higher than reported for idiotype vaccines, is below what can be achieved with a microbial vaccine. A higher frequency and magnitude of immune response to cancer vaccines will probably be required for a significant long-term clinical effect.
The 2-arm design of this study facilitated an assessment of the safety/toxicity profile of the hTERT/survivin immunizations and the vaccine-primed T cells. Toxicity profiles were similar between the 2 arms except that a significant increase in diarrhea was observed among the patients in arm A (P = .001). Some of these cases of diarrhea (7 of 54 patients; 13%) were documented by colonic biopsy to be because of grade I-III autologous GVHD, but these events were evenly distributed between the 2 arms. The reason for the excess cases of diarrhea in the patients in arm A is unclear, but both hTERT and survivin are known to be expressed in colonic crypt epithelial cells, suggesting a possible immunologic basis for this observation.46,47 Nonetheless, our data did not show evidence to correlate adverse effects such as diarrhea to hTERT/survivin tetramer responses. The pattern of hTERT/survivin vaccine reactivities showed no severe reactions. A significant increase in the frequency of > 50 mm of induration/redness developed during the course of immunizations consistent with a DTH response that was documented by immunoassays on a deep skin biopsy taken from 1 patient.
We anticipated that patients positive for HLA-A2 who received the hTERT/survivin vaccine (arm A) might show better myeloma control. However, patients in arm A exhibited an inferior EFS compared with patients in arm B with no difference in OS. However, the inferiority in EFS is unlikely because of the hTERT/survivin vaccine because only approximately one-third of patients in arm A developed an immune response. Furthermore, several differences in patient and treatment-related factors between the 2 arms probably contributed to a higher relapse rate in the arm A cohort: (1) the percentage of patients with abnormal cytogenetics was 50% in arm A but 27% in arm B (P = .14); (2) the percentage of plasma cells in the marrow at enrollment was 32% in arm A compared with 22% in arm B; and, most importantly, (3) the percentage of eligible patients who received thalidomide maintenance was 29% in arm A and 77% in arm B (P = .003). After adjustment for the difference in thalidomide usage alone, the difference in EFS between arms A and B was no longer significant. The reason for differential thalidomide usage was that the study site where most of the patients in arm A were enrolled did not routinely use thalidomide maintenance after transplantation as a matter of practice. As the standard of care has evolved to include maintenance after transplantation, future studies should mandate such therapy for all patients. Emerging data suggest that lenalidomide after transplantation could play a dual role as a maintenance treatment for myeloma and an immune modulator with the potential to augment or sustain cellular and antibody responses to tumor vaccines.11,12,48
In summary, this study shows for the first time that adoptive transfer of vaccine-primed and costimulated autologous T cells generates a rapid and schedule-dependent recovery of the cellular and humoral immune system in patients with myeloma and that immune responses to a cancer vaccine occur in a substantial proportion of patients early after autotransplantation. Whether T-cell transfers /or tumor antigen immunizations or both enhanced EFS in this study is unknown. However, by a number of measures, the magnitude of the immune recovery was associated with improved EFS. Our results after the therapeutic induction of rapid lymphocyte recovery are consistent with previous studies showing that unmanipulated lymphocyte levels in patients with myeloma correlate to EFS.13-16 Future studies will aim to improve the immunotherapeutic response by exploring vaccines for other tumor antigens (eg, cancer/testis antigens) and by incorporating more potent adjuvants.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the apheresis centers and nurses of the BMT programs of the University of Maryland Greenebaum Cancer Center and the Abramson Cancer Center for outstanding clinical care provided to our patients. We specifically thank Sandra Westphal, Kathleen Ruehle, RN, and Carolynn Harris for cell processing, patient coordination, and study regulatory support at the University of Maryland site. We thank Yiping Liu at the University of Calgary for providing posttransplantation peripheral blood samples from a cohort of transplant recipients who did not receive T-cell transfers for comparative analyses.
This work was supported by the National Institutes of Health (grant 5R21CA130293-02, A.P.R. and grant 5K23A10675670-5, N.A.A.), by an ARRA supplemental grant (A.P.R.), by the Leukemia & Lymphoma Society (grant 7414-07; C.H.J., B.L.L., and R.H.V.), and by Beckman Foundation Award (R.H.V.).
National Institutes of Health
Authorship
Contribution: A.P.R. designed the research, performed the research, analyzed the data, and wrote the paper; N.A.A. performed the research and helped write the paper; E.A.S. designed the research, performed the research, and helped write the paper; D.T.V., S.J., E.V., M.F.P.,. G.A., A.B.,. S.Y., A.C., S.P., H.M., R.B., Z.Z., T.M., J.C., and A.C. performed research; H.-B.F., L.C., and M.T.T. performed statistical analysis; A.C. analyzed data; J.S. contributed vital reagents; R.H. Vonderheide designed research, performed research, contributed vital new reagents, and helped write the paper; B.L. Levine designed research, performed research, contributed vital reagents, analyzed data, and helped write the paper; C.H.J. designed the research, contributed vital new reagents, analyzed the data, and helped write the paper.
Conflict-of-interest disclosure: R.H.V. declares a potential financial conflict of interest related to inventorship on a patent regarding hTERT as a tumor-associated antigen for cancer immunotherapy. C.H.J. and B.L.L. have patents and patent applications in the field of adoptive immunotherapy but have been divested of financial benefit from this technology. This arrangement is under compliance with the policies of the University of Pennsylvania. The remaining authors declare no competing financial interests.
Correspondence: Aaron P. Rapoport, University of Maryland Greenebaum Cancer Center, University of Maryland School of Medicine and Department of Medicine, 22 S Greene St, Baltimore, MD 21201; e-mail: arapoport@umm.edu.
References
Author notes
A.P.R., N.A.A., E.A.S., B.L.L., R.H.V., and C.H.J. contributed equally to this study.
R.H.V. and C.H.J. shared senior authorship.