Abstract
The success of reduced intensity conditioning (RIC) transplantation is largely dependent on alloimmune effects. It is critical to determine whether immune modulation with anti–T-cell antibody infusion abrogates the therapeutic benefits of transplantation. We examined 1676 adults undergoing RIC transplantation for hematologic malignancies. All patients received alkylating agent plus fludarabine; 792 received allografts from a human leukocyte antigen-matched sibling, 884 from a 7 or 8 of 8 HLA-matched unrelated donor. Using Cox regression, outcomes after in vivo T-cell depletion (n = 584 antithymocyte globulin [ATG]; n = 213 alemtuzumab) were compared with T cell– replete (n = 879) transplantation. Grade 2 to 4 acute GVHD was lower with alemtuzumab compared with ATG or T cell– replete regimens (19% vs 38% vs 40%, P < .0001) and chronic GVHD, lower with alemtuzumab, and ATG regimens compared with T-replete approaches (24% vs 40% vs 52%, P < .0001). However, relapse was more frequent with alemtuzumab and ATG compared with T cell–replete regimens (49%, 51%, and 38%, respectively, P < .001). Disease-free survival was lower with alemtuzumab and ATG compared with T cell–replete regimens (30%, 25%, and 39%, respectively, P < .001). Corresponding probabilities of overall survival were 50%, 38%, and 46% (P = .008). These data suggest adopting a cautious approach to routine use of in vivo T-cell depletion with RIC regimens.
Introduction
The use of reduced intensity conditioning (RIC) for allogeneic hematopoietic stem cell transplantation increased steadily in the past decade and now accounts for 40% of allogeneic transplants for hematologic malignancies in adults. Anti–T-cell antibody infusions (alemtuzumab or antithymocyte globulin [ATG] preparations) are often used as a component of conditioning to both promote engraftment and to diminish GVHD.1,2 No large prospective randomized trials assessing the overall efficacy of this strategy have been undertaken in the RIC setting. The success of RIC transplantation relies on the integrity of graft-versus-tumor activity because the cytoreductive effects of RIC are usually insufficient to eradicate malignancy. It is therefore critical to understand the impact of anti–T-cell agents because it is possible that they might abrogate the therapeutic benefits of the graft in this setting.
To examine this issue, we evaluated the outcome of RIC transplantation in 1676 patients transplanted between 2000 and 2007 for a hematologic malignancy and reported to the Center for International Blood and Marrow Transplant Research. Of these, 797 patients received conditioning that included anti–T-cell antibodies (n = 584 ATG, n = 213 alemtuzumab), whereas 879 patients received no in vivo T-cell depletion (T cell–replete regimens). We assessed impact of anti–T-cell antibody therapy on acute and chronic GVHD, relapse rates, nonrelapse mortality, disease-free survival, and overall survival
Methods
Collection of data
Data on transplantations were obtained from the Center for International Blood and Marrow Transplant Research, a voluntary group of more than 450 transplant centers worldwide that contribute data prospectively on consecutive transplantations performed at each transplant center to a Statistical Center at the Medical College of Wisconsin, Milwaukee, WI. Patients are followed longitudinally annually. Computerized error checks, physician review of data, and on-site audits ensure data quality. A total of 164 transplant centers contributed patients, and all transplantations were performed in 2000 to 2007. This study was approved by the Institutional Review Board of the Medical College of Wisconsin (HRRC# 056-87).
Inclusion criteria
Patients were 21 to 69 years of age with acute lymphoblastic leukemia, acute myeloid leukemia, chronic myeloid leukemia, myelodysplastic syndrome, chronic lymphocytic leukemia, non-Hodgkin lymphoma, and Hodgkin lymphoma. Patients received allografts from an HLA-matched sibling or an adult unrelated donor matched at the allele-level at HLA-A, -B, -C, -DRB1 (8 of 8 HLA-matched) or mismatched at a single locus (7 of 8 HLA-matched), the accepted standard for these graft types.3 A total of 29% of patients with non-Hodgkin lymphoma and 88% of patients with Hodgkin lymphoma received prior autologous transplantation. None of the patients had received a prior allogeneic transplant. All patients received fludarabine plus an alkylating agent (cyclophosphamide, melphalan, or busulfan). RIC was defined as melphalan dose ≤ 140 mg/m2, busulfan ≤ 8 mg/kg, and cyclophosphamide < 120 mg/kg.4 Patients receiving low-dose total body irradiation were excluded as only a small fraction of these patients received in vivo T-cell depletion. Recipients of in vitro T cell–depleted grafts were excluded.
End points
Neutrophil recovery was defined as achieving an absolute neutrophil count of ≥ 0.5 × 109/L for 3 consecutive days; and platelet recovery as achieving platelets ≥ 20 × 109/L, unsupported by transfusion for 7 days. Secondary graft failure was defined as sustained loss of absolute neutrophil count of ≥ 0.5 × 109/L after initial recovery in the absence of recurrent disease. Incidences of grade 2 to 4 acute and chronic GVHD were based on reports from each transplant center using standard criteria.5,6 Nonrelapse mortality was defined as death occurring in continuous complete remission, and relapse was defined as disease recurrence based on morphologic evaluation supported by reappearance of abnormalities in cytogenetic or molecular analyses or radiographic progression (an increase in size of sites of disease; ≥ 25% increase in largest diameter). Disease-free survival was defined as survival in a state of continuous complete remission.
Statistical analysis
Patients were considered in 3 groups: those receiving ATG, those receiving alemtuzumab, and those receiving no anti–T-cell therapy. Variables related to patients, disease, and transplantation (Table 1) were compared among the 3 groups using the χ2 statistic. The probabilities of disease-free and overall survival were calculated with the Kaplan-Meier estimator.7 Probabilities of neutrophil and platelet recovery, acute and chronic GVHD, nonrelapse mortality, and relapse were calculated with the cumulative incidence estimator to accommodate competing risks.7 For neutrophil and platelet recovery and acute and chronic GVHD, death without the event was the competing event. For nonrelapse mortality, relapse was the competing event, and for relapse, nonrelapse mortality was the competing event. For analysis of disease-free survival, relapse or death from any cause (ie, treatment failure) was considered an event. For overall survival, death from any cause was considered an event. In all analyses, data on patients without an event were censored at last follow-up.
Patient, disease, and transplantation characteristics
| . | ATG, no. (%) . | Alemtuzumab, no. (%) . | T cell–replete, no. (%) . | P . |
|---|---|---|---|---|
| No. of patients | 584 | 213 | 879 | |
| Age, y | .16 | |||
| 21-29 | 39 (7) | 9 (4) | 54 (6) | |
| 30-39 | 59 (10) | 27 (13) | 91 (10) | |
| 40-49 | 92 (16) | 47 (22) | 179 (20) | |
| 50-59 | 235 (40) | 70 (33) | 342 (39) | |
| 60-69 | 159 (27) | 60 (28) | 213 (24) | |
| Disease | < .001 | |||
| Acute myeloid leukemia | 214 (37) | 55 (26) | 272 (31) | |
| Acute lymphoblastic leukemia | 18 (3) | 7 (3) | 39 (4) | |
| Chronic myeloid leukemia | 51 (9) | 9 (4) | 63 (7) | |
| Chronic lymphocytic leukemia | 58 (10) | 32 (15) | 99 (11) | |
| Myelodysplastic syndrome | 66 (11) | 13 (6) | 81 (9) | |
| Non-Hodgkin lymphoma* | 136 (23) | 85 (40) | 274 (31) | |
| Hodgkin lymphoma† | 41 (7) | 12 (6) | 51 (6) | |
| Disease status before transplantation | .28 | |||
| Early | 193 (33) | 58 (27) | 271 (31) | |
| Advanced | 391 (67) | 155 (73) | 608 (69) | |
| Performance score before transplantation | .08 | |||
| < 90%s | 188 (32) | 47 (22) | 264 (30) | |
| 90%-100% | 352 (60) | 135 (63) | 551 (63) | |
| Not reported | 44 (8) | 31 (15) | 64 (7) | |
| Conditioning regimen | < .001 | |||
| Busulfan + fludarabine | 375 (54) | 42 (20) | 300 (34) | |
| Melphalan + fludarabine | 132 (23) | 81 (38) | 310 (35) | |
| Cyclophosphamide+fludarabine | 77 (13) | 90 (42) | 269 (31) | |
| Graft type | .001 | |||
| Bone marrow | 84 (14) | 36 (17) | 83 (9) | |
| Peripheral blood progenitor cells | 500 (86) | 177 (83) | 796 (91) | |
| GVHD prophylaxis | < .001 | |||
| Cyclosporine alone | 68 (12) | 37 (17) | 95 (11) | |
| Cyclosporine + methotrexate | 91 (16) | 31 (15) | 243 (28) | |
| Cyclosporine + other agents | 79 (14) | 19 (9) | 103 (12) | |
| Tacrolimus alone | 30 (5) | 54 (25) | 11 (1) | |
| Tacrolimus + methotrexate | 242 (41) | 57 (27) | 329 (37) | |
| Tacrolimus + other agents | 74 (13) | 15 (7) | 98 (11) | |
| Donor-recipient CMV serostatus | < .001 | |||
| Donor and recipient seronegative | 161 (28) | 54 (25) | 188 (21) | |
| Donor seropositive/recipient seronegative | 71 (12) | 20 (9) | 85 (10) | |
| Donor seronegative/recipient seropositive | 156 (27) | 81 (38) | 198 (23) | |
| Donor and recipient seropositive | 185 (32) | 54 (25) | 361 (41) | |
| Unknown | 11 (2) | 4 (2) | 47 (5) | |
| Donor type | < .001 | |||
| HLA-matched sibling | 228 (39) | 47 (22) | 517 (59) | |
| Unrelated, 8/8 matched | 251 (43) | 121 (57) | 278 (32) | |
| Unrelated, 7/8 matched | 105 (18) | 45 (21) | 84 (10) | |
| Year of transplantation | < .001 | |||
| 2000-2003 | 176 (30) | 66 (31) | 353 (40) | |
| 2004-2007 | 408 (70) | 147 (69) | 526 (60) |
| . | ATG, no. (%) . | Alemtuzumab, no. (%) . | T cell–replete, no. (%) . | P . |
|---|---|---|---|---|
| No. of patients | 584 | 213 | 879 | |
| Age, y | .16 | |||
| 21-29 | 39 (7) | 9 (4) | 54 (6) | |
| 30-39 | 59 (10) | 27 (13) | 91 (10) | |
| 40-49 | 92 (16) | 47 (22) | 179 (20) | |
| 50-59 | 235 (40) | 70 (33) | 342 (39) | |
| 60-69 | 159 (27) | 60 (28) | 213 (24) | |
| Disease | < .001 | |||
| Acute myeloid leukemia | 214 (37) | 55 (26) | 272 (31) | |
| Acute lymphoblastic leukemia | 18 (3) | 7 (3) | 39 (4) | |
| Chronic myeloid leukemia | 51 (9) | 9 (4) | 63 (7) | |
| Chronic lymphocytic leukemia | 58 (10) | 32 (15) | 99 (11) | |
| Myelodysplastic syndrome | 66 (11) | 13 (6) | 81 (9) | |
| Non-Hodgkin lymphoma* | 136 (23) | 85 (40) | 274 (31) | |
| Hodgkin lymphoma† | 41 (7) | 12 (6) | 51 (6) | |
| Disease status before transplantation | .28 | |||
| Early | 193 (33) | 58 (27) | 271 (31) | |
| Advanced | 391 (67) | 155 (73) | 608 (69) | |
| Performance score before transplantation | .08 | |||
| < 90%s | 188 (32) | 47 (22) | 264 (30) | |
| 90%-100% | 352 (60) | 135 (63) | 551 (63) | |
| Not reported | 44 (8) | 31 (15) | 64 (7) | |
| Conditioning regimen | < .001 | |||
| Busulfan + fludarabine | 375 (54) | 42 (20) | 300 (34) | |
| Melphalan + fludarabine | 132 (23) | 81 (38) | 310 (35) | |
| Cyclophosphamide+fludarabine | 77 (13) | 90 (42) | 269 (31) | |
| Graft type | .001 | |||
| Bone marrow | 84 (14) | 36 (17) | 83 (9) | |
| Peripheral blood progenitor cells | 500 (86) | 177 (83) | 796 (91) | |
| GVHD prophylaxis | < .001 | |||
| Cyclosporine alone | 68 (12) | 37 (17) | 95 (11) | |
| Cyclosporine + methotrexate | 91 (16) | 31 (15) | 243 (28) | |
| Cyclosporine + other agents | 79 (14) | 19 (9) | 103 (12) | |
| Tacrolimus alone | 30 (5) | 54 (25) | 11 (1) | |
| Tacrolimus + methotrexate | 242 (41) | 57 (27) | 329 (37) | |
| Tacrolimus + other agents | 74 (13) | 15 (7) | 98 (11) | |
| Donor-recipient CMV serostatus | < .001 | |||
| Donor and recipient seronegative | 161 (28) | 54 (25) | 188 (21) | |
| Donor seropositive/recipient seronegative | 71 (12) | 20 (9) | 85 (10) | |
| Donor seronegative/recipient seropositive | 156 (27) | 81 (38) | 198 (23) | |
| Donor and recipient seropositive | 185 (32) | 54 (25) | 361 (41) | |
| Unknown | 11 (2) | 4 (2) | 47 (5) | |
| Donor type | < .001 | |||
| HLA-matched sibling | 228 (39) | 47 (22) | 517 (59) | |
| Unrelated, 8/8 matched | 251 (43) | 121 (57) | 278 (32) | |
| Unrelated, 7/8 matched | 105 (18) | 45 (21) | 84 (10) | |
| Year of transplantation | < .001 | |||
| 2000-2003 | 176 (30) | 66 (31) | 353 (40) | |
| 2004-2007 | 408 (70) | 147 (69) | 526 (60) |
N = 405 received rabbit ATG; n = 148 received < 6 mg/kg total dose; n = 110 received 6-8 mg/kg, and n = 147 received > 8 mg/kg. N = 160 received horse ATG; n = 27 received < 30 mg/kg total dose; n = 93 received 30-50 mg/kg, and n = 40 received > 50 mg/kg.
CMV indicates cytomegalovirus.
N = 142 (29%) patients had prior autologous transplantation.
N = 92 (88%) patients had prior autologous transplantation.
Cox proportional hazard regression models were constructed for acute and chronic GVHD, nonrelapse mortality, relapse, disease-free survival, and overall survival.8 Multivariate models were built with the use of forward stepwise selection procedures and confirmed with the use of backward stepwise selection procedure. All variables significant at level ≤ .05 were included in the final models. Proportional-hazards assumption was tested for each variable individually, and all variables met this assumption.
The primary objective of the current analysis was to describe the overall impact of in vivo T-cell depletion (alemtuzumab or ATG) on the outcome of RIC transplantation and to evaluate the hypothesis that anti–T-cell antibody infusions might abrogate the therapeutic benefits of transplantation. Therefore, we compared transplant outcomes after in vivo T cell–depleted and T cell–replete transplants. Results are expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). The variable for in vivo T-cell depletion (alemtuzumab vs ATG vs T cell–replete) was held in all steps of model building. Other variables considered were patient age (< 50 vs 50-59 vs 60-69 years), performance score (90-100 vs < 90), disease (acute myeloid leukemia/myelodysplastic syndrome vs chronic myeloid leukemia vs chronic lymphocytic leukemia/follicular/mantle cell lymphoma vs acute lymphoblastic leukemia/Hodgkin lymphoma/other non-Hodgkin lymphoma), disease status at transplantation (first complete remission [early disease] vs subsequent remission or not in remission at transplantation [advanced disease]), donor (HLA-matched sibling vs 8 of 8 HLA-matched unrelated donor vs 7 of 8 HLA-matched unrelated donor), graft type (bone marrow vs peripheral blood progenitor cells), conditioning regimen (cyclophosphamide vs busulfan vs melphalan regimens), GVHD prophylaxis (tacrolimus/cyclosporine alone vs tacrolimus/cyclosporine plus methotrexate vs tacrolimus/cyclosporine + other agents [not methotrexate]), donor-recipient cytomegalovirus serostatus (donor/recipient seronegative vs donor seropositive/recipient seronegative vs donor seronegative/recipient seropositive vs donor/recipient seropositive), and transplant year (2000-2003 vs 2004-2007). Individual covariates were entered as categorical variables (shown above). There were no significant interactions between the variable for in vivo T-cell depletion and other significant variables in the final model. The effect of acute and chronic GVHD on relapse was explored by entering them as time-dependent covariates in the Cox proportional hazards models. There were no significant center effects detected using the random effect model.9 All P values are 2-sided. Analyses were done with SAS software (Version 9.2).
Results
Patient, disease, and transplantation characteristics
Characteristics of patients, their diseases, and transplantations are shown in Table 1. Of the 1676 eligible patients, 48% received in vivo T-cell depletion (ATG n = 584, 35%; alemtuzumab n = 213, 13%). Patients in the 3 treatment groups were similar with respect to their age, performance score, and disease status at transplantation. The median age of patients was 54 years, and approximately 60% of patients in each of the treatment groups had performance scores of 90 to 100. One-third of patients in all treatment groups were transplanted in first complete remission. Peripheral blood progenitor cell was the predominant graft type in all treatment groups. The median follow-up of surviving patients was 3 years.
There were some differences in transplantation characteristics among the 3 treatment groups. In vivo T-cell depletion was more commonly used with unrelated donor transplantations (59%) than with HLA-matched sibling donor transplantations (35%). Alemtuzumab was used more frequently for lymphoid malignancies. Patients who received ATG were more likely to receive busulfan-containing conditioning regimens, whereas patients who received alemtuzumab were more likely to receive melphalan- or cyclophosphamide-containing regimens. All conditioning regimens contained fludarabine in addition to the alkylating agent. Tacrolimus-containing GVHD prophylaxis regimens were more common with in vivo T-cell depletion.
The median total dose of alemtuzumab was 60 mg. Among patients who received ATG, approximately 70% of recipients received rabbit ATG (median dose, 7 mg/kg) and 27%, horse ATG (median dose, 40 mg/kg). The type of ATG administered was not reported for 3% of patients.
Hematopoietic recovery
The 28-day probabilities of neutrophil recovery were similar in recipients of T cell–replete, alemtuzumab-containing, and ATG-containing regimens (96%, 95% CI, 95%-97%; 95%, 95% CI, 93%-96%; and 94%, 95% CI, 91%-97%, respectively). Compared with T cell–replete regimens (92%, 95% CI, 91%-93%), the day 60 probabilities of platelet recovery were lower with alemtuzumab-containing regimens (89%, 95% CI, 86%-92%, P = .08) and with ATG-containing regimens (88%, 95% CI, 86%-91%, P = .004), although the difference was statistically significant only for the ATG group. Secondary graft failure in the absence of recurrent disease was uncommon and occurred in only 27 patients (T cell–replete group, n = 11 of 861 [1%]; alemtuzumab group, n = 7 of 204 [3%]; ATG group, n = 9 of 561 [2%]).
GVHD
Results of univariate and multivariate analyses are shown in Table 2. Compared with T cell–replete regimens, day 100 rates of grades 2 to 4 and grades 3 to 4 acute GVHD were lower with alemtuzumab-containing regimens; 40% (95% CI, 37%-43%) vs 19% (95% CI, 14%-25%), P < .001 and 22% (95% CI, 20%-25%) vs 11% (95% CI, 7%-16%), P < .001, respectively. Rates of grades 2 to 4 and grades 3 to 4 acute GVHD were similar with T-cell-replete and ATG-containing regimens: 40% (95% CI, 37%-43%) vs 38% (95% CI, 34%-42%) and 22% (95% CI, 20%-25%) vs 21% (95% 17%-24%), respectively. The risk of developing grades 2 to 4 and 3 to 4 acute GVHD was lower with alemtuzumab-containing compared with ATG-containing regimens (P < .001).
Results of univariate analysis and multivariate analysis for acute GVHD, chronic GVHD, nonrelapse mortality, relapse, disease-free survival, and overall survival
| Outcome . | HR (95% CI) . | P . |
|---|---|---|
| Univariate analysis | ||
| Grade 2-4 acute GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.98 (0.83-1.16) | .82 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.43 (0.31-0.59) | < .0001 |
| ATG regimen vs alemtuzumab regimen | 2.29 (1.66-3.16) | < .0001 |
| Grade 3-4 acute GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.94 (0.75-1.72) | .58 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.50 (0.33-0.75) | .0008 |
| ATG regimen vs alemtuzumab regimen | 1.89 (1.24-2.89) | .003 |
| Chronic GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.72 (0.62-0.85) | < .0001 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.39 (0.29-0.52) | < .0001 |
| ATG regimen vs alemtuzumab regimen | 1.87 (1.38-2.52) | < .0001 |
| Non-relapse mortality | ||
| ATG regimen vs T cell–replete regimen | 1.38 (1.12-1.71) | .003 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.09 (0.79-1.51) | .60 |
| ATG regimen vs alemtuzumab regimen | 1.27 (0.91-1.78) | .17 |
| Relapse | ||
| ATG regimen vs T cell–replete regimen | 1.56 (1.33-1.83) | < .0001 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.60 (1.29-1.99) | < .0001 |
| ATG regimen vs alemtuzumab regimen | 0.97 (0.78-1.21) | .80 |
| Treatment failure (inverse of disease-free survival) | ||
| ATG regimen vs T cell–replete regimen | 1.49 (1.31-1.70) | < .0001 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.41 (1.18-1.70) | .0002 |
| ATG regimen vs alemtuzumab regimen | 1.06 (0.88-1.27) | .55 |
| Overall mortality | ||
| ATG regimen vs T cell–replete regimen | 1.34 (1.17-1.54) | < .0001 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.10 (0.90-1.35) | .35 |
| ATG regimen vs alemtuzumab regimen | 1.22 (0.99-1.50) | .07 |
| Multivariate analysis | ||
| Grade 2-4 acute GVHD* | ||
| ATG regimen vs T cell–replete regimen | 0.88 (0.74-1.04) | .12 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.33 (0.24-0.46) | < .001 |
| ATG regimen vs alemtuzumab regimen | 2.66 (1.91-3.70) | < .001 |
| Grade 3-4 acute GVHD† | ||
| ATG regimen vs T cell–replete regimen | 0.86 (0.69-1.08) | .19 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.42 (0.27-0.64) | < .001 |
| ATG regimen vs alemtuzumab regimen | 2.08 (1.34-3.21) | < .001 |
| Chronic GVHD‡ | ||
| ATG regimen vs T cell–replete regimen | 0.69 (0.59-0.81) | < .001 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.34 (0.26-0.46) | < .001 |
| ATG regimen vs alemtuzumab regimen | 2.01 (1.49-2.72) | < .001 |
| Non-relapse mortality§ | ||
| ATG regimen vs T cell–replete regimen | 1.34 (1.07-1.67) | .01 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.04 (0.72-1.49) | .85 |
| ATG regimen vs alemtuzumab regimen | 1.29 (0.89-1.86) | .18 |
| Relapse‖ | ||
| ATG regimen vs T cell–replete regimen | 1.53 (1.29-1.81) | < .001 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.54 (1.24-1.93) | < .001 |
| ATG regimen vs alemtuzumab regimen | 0.99 (0.78-1.26) | .940 |
| Treatment failure (inverse of disease-free survival)¶ | ||
| ATG regimen vs T cell–replete regimen | 1.46 (1.29-1.66) | < .0001 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.40 (1.17-1.68) | .0003 |
| ATG regimen vs alemtuzumab regimen | 1.04 (0.86-1.27) | .67 |
| Overall mortality# | ||
| ATG regimen vs T cell–replete regimen | 1.25 (1.09-1.44) | .002 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.09 (0.87-1.36) | .46 |
| ATG regimen vs alemtuzumab regimen | 1.15 (0.92-1.44) | .22 |
| Outcome . | HR (95% CI) . | P . |
|---|---|---|
| Univariate analysis | ||
| Grade 2-4 acute GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.98 (0.83-1.16) | .82 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.43 (0.31-0.59) | < .0001 |
| ATG regimen vs alemtuzumab regimen | 2.29 (1.66-3.16) | < .0001 |
| Grade 3-4 acute GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.94 (0.75-1.72) | .58 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.50 (0.33-0.75) | .0008 |
| ATG regimen vs alemtuzumab regimen | 1.89 (1.24-2.89) | .003 |
| Chronic GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.72 (0.62-0.85) | < .0001 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.39 (0.29-0.52) | < .0001 |
| ATG regimen vs alemtuzumab regimen | 1.87 (1.38-2.52) | < .0001 |
| Non-relapse mortality | ||
| ATG regimen vs T cell–replete regimen | 1.38 (1.12-1.71) | .003 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.09 (0.79-1.51) | .60 |
| ATG regimen vs alemtuzumab regimen | 1.27 (0.91-1.78) | .17 |
| Relapse | ||
| ATG regimen vs T cell–replete regimen | 1.56 (1.33-1.83) | < .0001 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.60 (1.29-1.99) | < .0001 |
| ATG regimen vs alemtuzumab regimen | 0.97 (0.78-1.21) | .80 |
| Treatment failure (inverse of disease-free survival) | ||
| ATG regimen vs T cell–replete regimen | 1.49 (1.31-1.70) | < .0001 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.41 (1.18-1.70) | .0002 |
| ATG regimen vs alemtuzumab regimen | 1.06 (0.88-1.27) | .55 |
| Overall mortality | ||
| ATG regimen vs T cell–replete regimen | 1.34 (1.17-1.54) | < .0001 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.10 (0.90-1.35) | .35 |
| ATG regimen vs alemtuzumab regimen | 1.22 (0.99-1.50) | .07 |
| Multivariate analysis | ||
| Grade 2-4 acute GVHD* | ||
| ATG regimen vs T cell–replete regimen | 0.88 (0.74-1.04) | .12 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.33 (0.24-0.46) | < .001 |
| ATG regimen vs alemtuzumab regimen | 2.66 (1.91-3.70) | < .001 |
| Grade 3-4 acute GVHD† | ||
| ATG regimen vs T cell–replete regimen | 0.86 (0.69-1.08) | .19 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.42 (0.27-0.64) | < .001 |
| ATG regimen vs alemtuzumab regimen | 2.08 (1.34-3.21) | < .001 |
| Chronic GVHD‡ | ||
| ATG regimen vs T cell–replete regimen | 0.69 (0.59-0.81) | < .001 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.34 (0.26-0.46) | < .001 |
| ATG regimen vs alemtuzumab regimen | 2.01 (1.49-2.72) | < .001 |
| Non-relapse mortality§ | ||
| ATG regimen vs T cell–replete regimen | 1.34 (1.07-1.67) | .01 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.04 (0.72-1.49) | .85 |
| ATG regimen vs alemtuzumab regimen | 1.29 (0.89-1.86) | .18 |
| Relapse‖ | ||
| ATG regimen vs T cell–replete regimen | 1.53 (1.29-1.81) | < .001 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.54 (1.24-1.93) | < .001 |
| ATG regimen vs alemtuzumab regimen | 0.99 (0.78-1.26) | .940 |
| Treatment failure (inverse of disease-free survival)¶ | ||
| ATG regimen vs T cell–replete regimen | 1.46 (1.29-1.66) | < .0001 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.40 (1.17-1.68) | .0003 |
| ATG regimen vs alemtuzumab regimen | 1.04 (0.86-1.27) | .67 |
| Overall mortality# | ||
| ATG regimen vs T cell–replete regimen | 1.25 (1.09-1.44) | .002 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.09 (0.87-1.36) | .46 |
| ATG regimen vs alemtuzumab regimen | 1.15 (0.92-1.44) | .22 |
Model adjusted for GVHD prophylaxis, donor source, and year of transplantation.
Model adjusted for GVHD prophylaxis, donor source, and year of transplantation.
Model adjusted for donor source and year of transplantation.
Model adjusted for age, performance score, conditioning regimen, GVHD, prophylaxis, and donor source.
Model adjusted for performance score, disease, disease status, and conditioning regimen.
Model adjusted for age, performance score, disease, and disease status.
Model adjusted for age, performance score, disease, disease status, GVHD prophylaxis, and donor source.
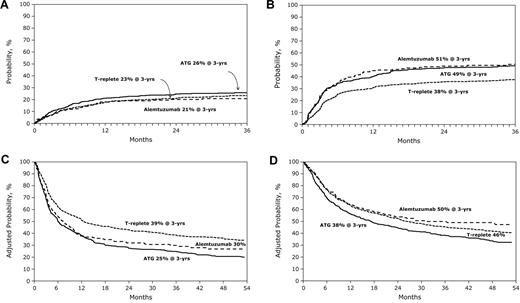
Compared with T cell–replete regimens (52%, 95% CI, 49%-56%), 3-year rates of chronic GVHD were lower with alemtuzumab-containing (24%, 95% CI, 18%-30%, P < .001) and ATG-containing (40%, 95% CI, 36%-44%, P < .001) regimens. The risk of developing chronic GVHD was also lower with alemtuzumab-containing compared with ATG-containing regimens (P < .001).
We also examined for differences in GVHD risks by type of ATG (rabbit vs horse). Compared with horse ATG, risks of grades 2 to 4 (HR = 0.73, P = .03) and grades 3 to 4 acute GVHD (HR = 0.62, P = .01) were lower with rabbit than horse ATG; there was no difference in chronic GVHD (HR = 1.03, P = .85).
Nonrelapse mortality
Compared with T cell–replete regimens (23%, 95% CI, 21%-26%), 3-year rates of nonrelapse mortality were similar after alemtuzumab-containing (21%, 95% CI, 15%-27%) but higher after ATG-containing (26%, 95% CI, 22%-30%) regimens (Table 2; Figure 1A). In ATG recipients, nonrelapse mortality risk was lower with rabbit ATG compared with horse ATG (HR = 0.60, P = .004).
Impact of anti–T-cell antibody infusion on outcome of RTC transplantation. (A) The 3-year probability of nonrelapse mortality after alemtuzumab-containing, ATG-containing, and T cell–replete transplant. (B) The 3-year probability of relapse after alemtuzumab-containing, ATG-containing, and T cell–replete transplants. (C) The 3-year probability of disease-free survival after alemtuzumab-containing, ATG-containing, and T cell–replete transplants after adjusting for patient age, performance score, disease, and disease status, the other significant factors. (D) The 3-year probability of overall survival after alemtuzumab-containing, ATG-containing, and T cell–replete transplants after adjusting for patient age, performance score, disease, disease status, donor type, and GVHD prophylaxis, the other significant factors.
Impact of anti–T-cell antibody infusion on outcome of RTC transplantation. (A) The 3-year probability of nonrelapse mortality after alemtuzumab-containing, ATG-containing, and T cell–replete transplant. (B) The 3-year probability of relapse after alemtuzumab-containing, ATG-containing, and T cell–replete transplants. (C) The 3-year probability of disease-free survival after alemtuzumab-containing, ATG-containing, and T cell–replete transplants after adjusting for patient age, performance score, disease, and disease status, the other significant factors. (D) The 3-year probability of overall survival after alemtuzumab-containing, ATG-containing, and T cell–replete transplants after adjusting for patient age, performance score, disease, disease status, donor type, and GVHD prophylaxis, the other significant factors.
Epstein-Barr virus posttransplant lymphoproliferative disease (EBV-PTLD) was more frequent with in vivo T-cell depletion. Only 2 patients in the T cell–replete group developed EBV-PTLD compared with 5 patients who received alemtuzumab and 12 patients who received ATG. The 3-year cumulative incidences of EBV-PTLD in the 3 treatment groups were 0.1%, 2%, and 2%, respectively (P = .005). Four deaths were attributed to EBV-PTLD; all of these deaths occurred in patients who had received ATG-containing regimens.
Relapse
Compared with patients who received T cell–replete regimens (38%, 95% CI, 35%-41%), 3-year rates of relapse were higher in patients who received alemtuzumab-containing (51%, 95% CI, 44%-58%) and ATG-containing (49%, 95% CI, 45%-54%) regimens (Table 2; Figure 1B). Relapse risks were similar after alemtuzumab and ATG-containing regimens. Relapse risks were higher after rabbit than after horse ATG (HR = 1.43, P = .02). As risks of acute and chronic GVHD were significantly lower with in vivo T-cell depletion, we investigated whether differences in relapse risk could be accounted for by differences in GVHD risks. When considered as a time-dependent covariate in the relapse models, chronic, but not acute, GVHD was associated with lower relapse risks (HR = 0.76, P = .02). However, even after adjusting for the effects of GVHD, in vivo T-cell depletion was associated with a higher risk of relapse.
A total of 175 patients received donor leukocyte infusion (DLI). DLI was administered more commonly in recipients of alemtuzumab-containing regimens. Seventy of 879 (8%) patients in the T cell–replete group received DLI compared with 51 of 213 (24%) of patients in the alemtuzumab group and 54 of 584 (9%) patients in the ATG group. A total of 160 patients received DLI for recurrent disease (T cell–replete group, n = 68; alemtuzumab group, n = 44; ATG group, n = 48). DLI was planned as part of the transplantation regimen in 3 patients; 2 patients received DLI as treatment for EBV-PTLD, and 1 patient received cytotoxic T-lymphocyte–enriched infusion for anticytomegalovirus activity. The remaining patients received DLI for mixed or falling chimerism in the absence of recurrent disease.
Disease-free survival
Compared with patients who received T cell–replete regimens, disease-free survival was lower in patients who received alemtuzumab-containing and ATG-containing regimens (Table 2); there was not a statistically significant difference in disease-free survival between the 2 T-cell depletion agents. The 3-year probabilities of disease-free survival, adjusted for age, performance score, disease, and disease status were 39% (95% CI, 37%-40%), 30% (95% CI, 28%-32%), and 25% 95% CI, 24%-26%), P < .001, after T cell–replete, alemtuzumab-containing, and ATG-containing regimens, respectively (Figure 1C). Disease-free survival was similar with horse and rabbit ATG; the lower nonrelapse mortality with rabbit ATG was negated by higher relapse. Recurrent disease was the most common cause of treatment failure in all treatment groups.
As different doses and sources of ATG were used by transplant centers, we examined outcome at doses above and below median dose administered for both rabbit- and horse-derived ATG. We did not observe differences in disease-free survival by ATG dose (< 7 mg/kg vs ≥ 7 mg/kg for rabbit ATG and < 40 mg vs ≥ 40 mg/kg for horse ATG). The timing of ATG may also impact transplant outcomes. We did not collect data on timing of ATG administration. This is a limitation in the study given that the half-life of the active species of ATG in vivo is about a week, and early versus late administration can affect the degree of in vivo T-cell depletion.10,11
Overall survival
Compared with T cell–replete regimens, overall mortality was similar after alemtuzumab-containing regimens but higher with ATG-containing regimens (Table 2). The 3-year probabilities of overall survival, adjusted for age, performance score, disease, disease status, GVHD prophylaxis, and donor source were 46% (95% CI, 44%-47%), 50% (95% CI, 46%-53%), and 38% (95% CI, 37%-40%), after T cell–replete, alemtuzumab-containing, and ATG-containing regimens, respectively (Figure 1D). The difference between T cell–replete and alemtuzumab-containing regimens was not statistically significant, whereas the difference between T cell–replete and ATG-containing was (P = .008).
Because DLI was administered to 20% of patients with recurrent disease after transplantation in the T cell–replete group compared with 40% of patients in the alemtuzumab group and 20% of patients in the ATG group, we investigated whether there were differences in mortality risks among patients with recurrent disease. Mortality risks were higher in patients with recurrent disease in the T cell–replete (HR = 1.42, P = .008) compared with patients in the alemtuzumab group. A similar trend was observed in the ATG group (HR = 1.30, P = .056).
There were no differences in causes of death after in vivo T-cell depletion and T cell–replete transplants. In all treatment groups, recurrent disease was the most common cause of death and accounted for approximately 45% of deaths. The proportion of patients dying from infection, interstitial pneumonitis, adult respiratory distress syndrome, organ failure, and GVHD were also similar across the treatment groups.
Donor source
In vivo T-cell depletion was more frequently used for unrelated donor than HLA-matched sibling transplants. We conducted a separate analysis of HLA-matched sibling and unrelated donor transplants, and results are shown in Table 3. These results are similar to the analysis with the exception that ATG was less effective in reducing acute GVHD in the unrelated donor than the related donor setting. More importantly, compared with patients receiving T cell–replete grafts, use of in vivo T-cell depletion with alemtuzumab or ATG was associated with inferior disease-free survival whether they received related or unrelated transplants.
HLA-matched sibling transplantations and unrelated donor transplantations: results of multivariate analysis for acute GVHD, chronic GVHD, nonrelapse mortality, relapse, disease-free survival, and overall survival
| Outcome . | HR (95% CI) . | P . |
|---|---|---|
| HLA-matched sibling transplantations | ||
| Grade 2-4 acute GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.77 (0.58-1.02) | .06 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.34 (0.16-0.69) | .003 |
| Grade 3-4 acute GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.64 (0.44-0.94) | .02 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.49 (0.21-1.14) | .10 |
| Chronic GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.69 (0.54-0.90) | .006 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.34 (0.16-0.72) | .005 |
| Non-relapse mortality | ||
| ATG regimen vs T cell–replete regimen | 1.35 (0.97-1.88) | .08 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.82 (0.39-1.73) | .60 |
| Relapse | ||
| ATG regimen vs T cell–replete regimen | 1.35 (1.04-1.74) | .02 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.83 (1.14-2.91) | .01 |
| Treatment failure (inverse of disease-free survival) | ||
| ATG regimen vs T cell–replete regimen | 1.31 (1.07-1.60) | .008 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.47 (1.00-2.16) | .05 |
| Overall mortality | ||
| ATG regimen vs T cell–replete regimen | 1.28 (1.03-1.59) | .03 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.31 (0.85-2.01) | .23 |
| Unrelated donor transplantations | ||
| Grade 2-4 acute GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.97 (0.78-1.20) | .75 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.36 (0.25-0.52) | < .001 |
| Grade 3-4 acute GVHD | ||
| ATG regimen vs T cell–replete regimen | 1.02 (0.76-1.37) | .89 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.46 (0.28-0.77) | .003 |
| Chronic GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.68 (0.55-0.84) | < .001 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.34 (0.24-0.46) | < .001 |
| Non-relapse mortality | ||
| ATG regimen vs T cell–replete regimen | 1.56 (1.13-2.14) | .007 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.12 (0.72-1.74) | .61 |
| Relapse | ||
| ATG regimen vs T cell–replete regimen | 1.45 (1.15-1.83) | .002 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.42 (1.07-1.90) | .02 |
| Treatment failure (inverse of disease-free survival) | ||
| ATG regimen vs T cell–replete regimen | 1.50 (1.25-1.78) | < .001 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.29 (1.03-1.61) | .03 |
| Overall mortality | ||
| ATG regimen vs T cell–replete regimen | 1.25 (1.03-1.52) | .02 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.02 (0.78-1.34) | .89 |
| Outcome . | HR (95% CI) . | P . |
|---|---|---|
| HLA-matched sibling transplantations | ||
| Grade 2-4 acute GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.77 (0.58-1.02) | .06 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.34 (0.16-0.69) | .003 |
| Grade 3-4 acute GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.64 (0.44-0.94) | .02 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.49 (0.21-1.14) | .10 |
| Chronic GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.69 (0.54-0.90) | .006 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.34 (0.16-0.72) | .005 |
| Non-relapse mortality | ||
| ATG regimen vs T cell–replete regimen | 1.35 (0.97-1.88) | .08 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.82 (0.39-1.73) | .60 |
| Relapse | ||
| ATG regimen vs T cell–replete regimen | 1.35 (1.04-1.74) | .02 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.83 (1.14-2.91) | .01 |
| Treatment failure (inverse of disease-free survival) | ||
| ATG regimen vs T cell–replete regimen | 1.31 (1.07-1.60) | .008 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.47 (1.00-2.16) | .05 |
| Overall mortality | ||
| ATG regimen vs T cell–replete regimen | 1.28 (1.03-1.59) | .03 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.31 (0.85-2.01) | .23 |
| Unrelated donor transplantations | ||
| Grade 2-4 acute GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.97 (0.78-1.20) | .75 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.36 (0.25-0.52) | < .001 |
| Grade 3-4 acute GVHD | ||
| ATG regimen vs T cell–replete regimen | 1.02 (0.76-1.37) | .89 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.46 (0.28-0.77) | .003 |
| Chronic GVHD | ||
| ATG regimen vs T cell–replete regimen | 0.68 (0.55-0.84) | < .001 |
| Alemtuzumab regimen vs T cell–replete regimen | 0.34 (0.24-0.46) | < .001 |
| Non-relapse mortality | ||
| ATG regimen vs T cell–replete regimen | 1.56 (1.13-2.14) | .007 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.12 (0.72-1.74) | .61 |
| Relapse | ||
| ATG regimen vs T cell–replete regimen | 1.45 (1.15-1.83) | .002 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.42 (1.07-1.90) | .02 |
| Treatment failure (inverse of disease-free survival) | ||
| ATG regimen vs T cell–replete regimen | 1.50 (1.25-1.78) | < .001 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.29 (1.03-1.61) | .03 |
| Overall mortality | ||
| ATG regimen vs T cell–replete regimen | 1.25 (1.03-1.52) | .02 |
| Alemtuzumab regimen vs T cell–replete regimen | 1.02 (0.78-1.34) | .89 |
Disease types
Approximately 70% of the study population included patients with acute myeloid leukemia, myelodysplastic syndrome, and non-Hodgkin lymphoma. We performed 2 separate subset analysis that examined the effect of in vivo T-cell depletion for these patients and observed results consistent with the main analysis (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Discussion
The results of this analysis of 1676 patients suggest that the use of in vivo T-cell depletion with RIC regimens reduces chronic GVHD but significantly increases the likelihood of disease relapse, which negatively impacts disease-free survival. The finding of significantly higher relapse rates with in vivo T-cell depletion differs from reports of other much smaller studies.12-15 The higher relapse rate and lower disease-free survival were observed with both alemtuzumab-containing and ATG-containing regimens after both related and unrelated donor transplantation. Both anti T–cell agents were associated with lower rates of chronic GVHD; alemtuzumab also lowered the risk of acute GVHD. ATG-containing, but not alemtuzumab-containing, regimens were associated with lower survival. DLI was used as salvage for recurrent disease in a higher proportion of patients in the alemtuzumab group, and a higher proportion of patients in this group had non-Hodgkin lymphoma. Disease type and use of DLI for posttransplantation recurrence may have led to the observed survival advantage in recipients of alemtuzumab-containing regimens compared with recipients of ATG-containing regimens.
Both extent and specificity of T-cell depletion are likely to have an impact on GVHD, infectious complications, relapse, and survival. Consequently, the dose, schedule, and antibody source of each product might influence outcome. Although differences were noted between horse and rabbit sources of ATG in the rates of nonrelapse mortality and relapse, there was no difference in disease-free or overall survival rates between the 2; therefore, the 2 products were considered together in our primary model. An early report of rabbit ATG dose (thymoglobulin) suggested that a total dose of 15 mg/kg was associated with lower grade 3 to 4 acute and chronic GVHD but without a survival advantage because of the higher incidence of serious infections.16 A more recent report suggests that lowering the dose of rabbit ATG to 6 mg/kg from 7.5 mg/kg results in comparable rates of acute grades 2 to 4 and 3 to 4 GVHD and significantly lower rates of cytomegalovirus activation, bacterial infection, and nonrelapse mortality.15 We did not observe statistically significant differences in nonrelapse mortality or overall survival by dose or type of ATG. Data on alemtuzumab dose de-escalation in the RIC setting indicate that reduced levels of this agent can still protect against GVHD, although there is probably a threshold dose below which this effect ceases to exist.17,18
In the myeloablative setting, Finke et al compared in vivo T-cell depletion with an ATG preparation (Fresenius) in 202 patients with hematologic malignancies in a prospective randomized trial.19 In this study, acute GVHD and chronic GVHD were lower with ATG. Relapse and nonrelapse mortality rates were similar in the 2 cohorts, and there were no significant differences in overall survival rates between ATG recipients and those that did not receive ATG. It is possible that the cytotoxic effects of myeloablative conditioning blunted the impact of in vivo T-cell depletion on relapse. Indeed, if a strategy could diminish chronic GVH without compromising overall or disease-free survival, it would provide an important advance for transplant recipients.
To our knowledge, there is not a randomized clinical trial comparing in vivo T-cell depletion with T cell–replete transplants with RIC conditioning regimens. In a retrospective analysis of 155 patients with lymphoproliferative disorders who received alemtuzumab plus cyclosporine or cyclosporine plus methotrexate, acute GVHD and chronic GVHD were lower and cytomegalovirus activation higher with alemtuzumab. Similar to the findings in the current analysis, there were no differences in overall survival between the 2 treatment groups.20 Malladi et al also observed higher infection risks and lower chronic GVHD in 51 patients who received alemtuzumab compared with 37 patients who did not.21 We were unable to explore differences in cytomegalovirus reactivation between patients who received alemtuzumab and T cell–replete transplantations in the current analysis, but cytomegalovirus reactivation was not a more frequent cause of death in the alemtuzumab group. Unlike the current analysis, neither prior report observed differences in relapse.18,19 However, these studies were much smaller and so had much lower statistical power to detect such a difference.
The major limitation of this observational study is that choice of treatment strategy, including whether or not to use in vivo T-cell depletion, was at the discretion of the transplant center and therefore subject to bias. This analysis excluded recipients of low-dose total body irradiation conditioning regimens as only a small fraction of patients receiving these regimens also receive in vivo T-cell depletion. Although we performed a carefully controlled comparison of T cell–replete to in vivo T cell–depleted transplants that considered known prognostic factors, there may be several unknown or unmeasured factors that also contributed to the observed outcomes. Nevertheless, our data strongly suggest that, in recipients of RIC with an alkylating agent and fludarabine, in vivo T-cell depletion with anti–T-cell antibody preparations lowers the risk of chronic GVHD at the expense of increased relapse rates, resulting in inferior disease-free survival. An adverse effect on overall survival was seen with ATG-containing regimens. Our findings are concerning because in vivo T cell–depleting agents are a common component of RIC regimens and suggest that their benefits and risks should be further evaluated in a controlled setting, perhaps incorporating strategies to minimize the risk of relapse.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute, National Heart, Lung, and Blood Institute, National Institute of Allergy and Infectious Diseases (U24-CA76518), Health Resources and Services Administration (HHSH234200637015C), Office of Naval Research, and Department of Navy (N00014-08-1-1207). Clinical trials at M. D. Anderson Cancer Center (R.E.C.) for plerixafor and clofarabine are supported by Genzyme and for busulfan by Otsuka.
National Institutes of Health
Authorship
Contribution: R.J.S. and M.E. designed the study, interpreted results, and drafted the manuscript; J.L.R. and F.K. analyzed data and interpreted results; V.H., A.A., R.E.C., S.D., L.I., H.M.L., D.I.M., D.L.P., E.K.W., and M.M.H. critically reviewed and revised the manuscript; and all authors approved the final version.
Conflict-of-interest disclosure: R.J.S. is a consultant for Genzyme, Fresenius, and Miltenyi. R.E.C. is a consultant for Genzyme. E.K.W. is a member of the scientific advisory board for Genzyme and Fresenius. The remaining authors declare no competing financial interests.
Correspondence: Robert J. Soiffer, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: Robert_Soiffer@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal