Abstract
β-glucans have been reported to function as a potent adjuvant to stimulate innate and adaptive immune responses. However, β-glucans from different sources are differential in their structure, conformation, and thus biologic activity. Different preparations of β-glucans, soluble versus particulate, further complicate their mechanism of action. Here we show that yeast-derived particulate β-glucan activated dendritic cells (DCs) and macrophages via a C-type lectin receptor dectin-1 pathway. Activated DCs by particulate β-glucan promoted Th1 and cytotoxic T-lymphocyte priming and differentiation in vitro. Treatment of orally administered yeast-derived particulate β-glucan elicited potent antitumor immune responses and drastically down-regulated immunosuppressive cells, leading to the delayed tumor progression. Deficiency of the dectin-1 receptor completely abrogated particulate β-glucan–mediated antitumor effects. In contrast, yeast-derived soluble β-glucan bound to DCs and macrophages independent of the dectin-1 receptor and did not activate DCs. Soluble β-glucan alone had no therapeutic effect but significantly augmented antitumor monoclonal antibody-mediated therapeutic efficacy via a complement activation pathway but independent of dectin-1 receptor. These findings reveal the importance of different preparations of β-glucans in the adjuvant therapy and allow for the rational design of immunotherapeutic protocols usable in clinical trials.
Introduction
Effective cancer immunotherapy requires elicitation of potent antitumor T-cell responses and down-regulation of immunosuppressive components such as regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSCs).1 The utilization of proper adjuvants can augment T-cell responses quantitatively and qualitatively2 and modulates function of immunosuppressive cells. Even for antitumor monoclonal antibody (mAb)–based immunotherapy, addition of proper adjuvants can significantly increase monoclonal antibody-mediated therapeutic efficacy.3-5 As such adjuvants have been widely used in cancer immunotherapy such as with cancer vaccines to potentiate antitumor immune responses.6,7 Among all adjuvants, toll-like receptor (TLR) agonists and cytokines including GM-CSF have been tested in both preclinical and clinical settings for cancer treatment.8,9 However, some adjuvants may have tumor-promoting effects, for example, TLR4 ligation enhances immunosuppressive cytokine production by human lung cancer cells.10 High dose of GM-CSF has been shown to chemoattract MDSCs thus dampening the efficacy of cancer vaccines.11
In addition to TLR agonists and cytokine adjuvants, ligands for the RIG-I–like, the NOD and the dectin-1 receptors have been demonstrated to activate antigen-presenting cells (APCs) such as dendritic cells (DCs) and to promote innate and adaptive immune responses.12-14 Recent studies also demonstrate that β-glucans can function as potent adjuvants to stimulate innate and adaptive immune responses.13,15 β-glucans are glucose polymers that have a backbone of linear β-1,3-linked D-glucose molecules (β-1,3-D-glucan). They also have β-1,6-linked side chains of β1,3-D-glucan of varied sizes that occur at different intervals along the backbone.16 Because of the complexity of β-glucans and different methods performed in the studies, several molecules that could bind β-glucans have been reported on the membrane of macrophages, monocytes, neutrophils, NK cells, DCs, and some T cells. Thus far, the 4 kinds of receptors, complement receptor 3 (CR3, CD11b/CD18, αMβ2-integrin, Mac-1).17,18 Lactosylceramide (LacCer),19 selected scavenger receptors (SRs)20 including SR CD36,21 and dectin-114,22-24 have been identified as β-glucan receptors. In addition, TLR2 was also implicated in yeast zymosan β-glucan–induced cytokine production.25 However, it is unknown how each β-glucan receptor distinguishes its ligands. Another challenging issue in the β-glucan research area is that most studies used crude β-glucan extracts such as zymosan in which the β-glucan content was approximately 14%.26 Using crude glucan extracts generates more confusing data, and frequently, such data are contradictory.27,28 Furthermore, different preparations of β-glucans, soluble versus particulate, may also function differently in terms of their adjuvant effect.
Here, we used pure soluble and particulate β-glucans derived from the yeast Sacchromyces cerevisiae to determine their modulatory effect on innate and adaptive immune responses. We found that soluble and particulate β-glucans use differential receptors for eliciting their biologic activities. The dectin-1 pathway is essential for particulate β-glucan–mediated immuno modulatory effects on DC activation, macrophage phagocytosis, Th1, and cytotoxic T-lymphocyte (CTL) priming and differentiation, and in vivo antitumor immune responses. Soluble β-glucan alone does not have a direct stimulatory effect on adaptive T-cell responses but significantly augments antitumor monoclonal antibody-mediated therapeutic efficacy. This effect is not dependent on the dectin-1 pathway although complement activation is necessary and CR3+ neutrophils are effector cells. Thus, our findings demonstrate differential pathways required for adjuvant effects mediated by different preparations of yeast β-glucans.
Methods
Preparation of β-glucans
Both highly purified particulate β-glucan WGP and soluble poly-(1,6)-β-D-glucopyranosyl-(1,3)-β-D-glucopyranose (PGG) β-glucan isolated from the cell wall of S cerevisiae were provided by Biothera. The soluble PGG β-glucan is a pharmaceutical grade β-glucan with the purity > 99% and the particulate β-glucan WGP contains > 85% β-glucan, < 3.5% protein, < 0.01% manan, and < 8% moisture. For binding and phagocytosis assays, particulate β-glucan WGP was labeled with fluorescein dichlorotriazine (DTAF; Molecular Porbes-Invitrogen). DTAF-labeled soluble PGG β-glucan was provided by Biothera.
BMDC culture and thioglycollate-elicited macrophages
Flt-3 ligand-stimulated BMDCs were generated as described previously.15 Cultured BMDCs at day 7 were used for experiments. Peritoneal exudates and macrophages were collected after intraperitoneal injection of sterile 3% thioglycollate broth (BD Biosciences) for 4 days.
Phagocytosis and binding assays
For phagocytosis assay, a total of 5 × 105 BMDCs or thioglycollate-elicited macrophages were mixed with DTAF-labeled WGP (20 μg/mL) at 37°C for 1 hour and then washed with ice-cold PBS. Macrophages or BMDCs were identified by F4/80 or CD11c expression. The percentage of particulate β-glucan was determined by flow cytometry and the images were acquired by Imagestream (Amnis).
For binding assay, a total of 5 × 105 cells were mixed with DTAF-labeled PGG β-glucan (20 μg/mL) or DTAF-labeled WGP (20 μg/mL) on ice for 30 minutes. The percentage of binding was determined by flow cytometry. For inhibition assay, cells were mixed with soluble PGG β-glucan or dextran (200 μg/mL) on ice for 1 hour before the binding assay.
In vitro T-cell differentiation assay
CD4+ T cells from the lymph nodes and spleen of OT-II mice were purified with magnetic-activated cell-sorting beads (Miltenyi Biotec). Purified OT-II T cells (5 × 105) were cultured with BMDC (1 × 105) in the presence of 50 μg/mL of ovalbumin (OVA) protein with or without particulate β-glucan WGP (100 μg/mL) in complete RPMI 1640 culture medium containing 10% FBS supplemented with l-glutamine, sodium pyruvate, nonessential amino acids and 2-mercaptedthanol for 5 days. On day 5, the cells were restimulated with 50 ng/mL phorbol-12-nyristate-13-acetate (PMA), 500 ng/mL ionomycin (Sigma-Aldrich) in the presence of Golgiplug (BD Pharmingen). Cells were then surface stained with anti-CD4 or anti-CD8 mAbs, fixed, permeabilized and stained with anti–IL-17, anti–IFN-γ(Biolegend) mAbs. Data were acquired on a FACSCalibur flow cytometry (BD Immunocytometry Systems) and analyzed by FlowJo software Version 6 (TreeStar).
MUC1-transfected lymphoma RMA cells and ovalbumin-transfected mammary adenocarcinoma cell line
Lymphoma RMA cells transfected with human MUC1 was described previously.29 EO771 cells, a mammary adenocarcinoma cell line derived from a C57Bl/6J mouse, were from the Tumor Repository of the Division of Cancer Treatment, Diagnosis and Centers of the National Cancer Institute. EO771 cells were infected with pMiT-ovalbumin retrovirus in the presence of 10 μg/mL polybrene after 24 hour pretreatment with 250 ng tunicamycin (T7765; Sigma-Aldrich). Western blot was performed to confirm ovalbumin expression on the selected cell lines. To ensure tumorigenecity, an additional selection was made by in vivo passage of the transfected cells twice in C57BL/6 mice. The tumor cell lines with high ovalbumin expression levels and capable of generating subcutaneous tumors were used for experiments.
Mice and tumor models
Wildtype (WT) C57Bl/6 mice were purchased from the National Cancer Institute. C57Bl/6 C3-deficient (C3−/−) and CR3/CD11b−/− mice were from the Jackson laboratory. C57Bl/6 Dectin-1−/− mice were described previously.24 CD4 and CD8 ovalbumin T-cell receptor transgenic (Tg) OT-I and OT-II mice were purchased from Taconic. The murine tumor protocols were performed in compliance with all relevant laws and institutional guidelines and were approved by the Institutional Animal Care and use Committee of the University of Louisville.
For the EO771/ovalbumin tumor model, WT or Dectin-1−/− mice were implanted subcutaneously with EO771/ovalbumin cells (5 × 105/mouse). On day 8 after palpable tumors were formed, mice were treated with 200 μL of particulate β-glucan (4 mg/mL in PBS; total 800 μg) or 200 μL of phosphate-buffered saline given every day using an intragastric gavage needle. Tumor diameters were measured every third day and mice were killed when tumors reached 15 mm in diameter.
For the RAM-MUC1 tumor model, WT, Dectin-1−/−, or C3−/− mice were implanted subcutaneously with RAM-MUC1 cells (5 × 105/mouse). When the palpable tumors were formed, mice were treated with anti-MUC1 monoclonal antibody (BCP8, 200 μg/mouse) with or without soluble PGG β-glucan (1.2 mg/mouse) intravenously twice a week. Tumor diameters were measured every third day and mice were killed when tumors reached 15 mm in diameter. Mice were also monitored for survival. Granulocytes were depleted by treatment with the rat anti–mouse monoclonal antibody RB6-8C5 (anti–Gr-1) or isotype control monoclonal antibody, immediately before and during tumor therapy.
Surface and intracellular cytokine staining of tumor tissues, tumor DLNs and spleen
Tumor tissues were weighed and minced into small pieces followed by digestion with triple enzymes mixture containing collagenase type IV, hyaluronidase, and deoxyribonuclease for 45 minutes at 37°C on a rotating platform. Cells were washed twice with ice-cold phosphate-buffered saline. For surface marker staining, cells were first blocked with Fc-blocking monoclonal antibody for 15 minutes on ice and stained with mAbs including F4/80, CD11b, CD11c, Gr-1, CD8α, or CD4 (eBiosciences) on ice for 20 minutes, then washed and analyzed by flow cytometry.
For intracellular staining, tumor tissue single cell suspensions were stimulated with 50 ng/mL PMA, and 500 ng/mL ionomycin in the presence of Golgiplug. After 4 hours, cells were surface stained with anti-CD4 or anti-CD8 mAbs, fixed, permeabilized, and stained with anti–IL-17, anti–IFN-γ, and anti-Foxp3 mAbs.
To detect ovalbumin-specific CD8+ T-cell responses, single cell suspensions from tumors and tumor DLNs were stimulated with ovalbumin (100 μg/mL) for 3 days. On day 3, cells were restimulated with PMA plus ionomycin in the presence of Golgiplug and then the cells were surface stained with anti-CD8 monoclonal antibody and IFN-γ intracellular staining. To detect ovalbumin-specific CD8+ T-cell proliferation and IFN-γ production in the spleen, the splenocytes were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen). The labeled cells were cultured with ovalbumin (100 μg/mL) for 3 days. On day 3, cells were restimulated with PMA plus ionomycin and then surface stained with anti-CD8 monoclonal antibody and IFN-γ intracellular staining.
qRT-PCR
Tumor samples were treated with TRIzol reagent (Invitrogen) and total RNA was isolated and reverse-transcribed with TaqMan Reverse Transcription Reagents (Applied Biosystems). The indicated cytokine mRNA levels were quantified by quantitative RT-PCR (qRT-PCR) amplification using the BioRad MyiQ single color RT-PCR detection system. Briefly, complementary DNA was amplified in a 25 μL reaction mixture containing 12.5 μL of SYBR Green PCR supermix (BioRad), 100 ng of complementary DNA template, and selected primers (200 nM) using the recommended cycling conditions. The primer sequences, designed with primer Express software Version 2.0 (Applied Biosystems) are summarized in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Fluorescence-based neutrophil-mediated in vitro killing assay
The assay was performed as described previously.29 In brief, RMA-MUC1 tumor cells were mixed with anti-MUC1 monoclonal antibody (10 μg/mL) and fresh mouse serum as a complement source at 37°C for 30 minutes to obtain iC3b-opsonized tumor cells. The cells were washed extensively and labeled with a high concentration of CFSE (final concentration 7μM). The control RMA-MUC1 cells were incubated with anti-MUC1 monoclonal antibody but without serum, therefore no complement, and then labeled with a low concentration of CFSE (final concentration 0.7μM). The CFSE intensity was served as a reporter to discriminate iC3b-opsonized tumors (CFSEhigh) from nonopsonized tumor cells (CFSElow). Thioglycollate-elicited peritoneal neutrophils were isolated in ice-cold complete RPMI-1640 media from C57Bl/6 WT and CR3−/− mice 7 days after the intravenous administration of PGG soluble β-glucan (1.2 mg). Equal numbers of CFSEhigh cells and CFSElow cells were mixed together and incubated for 4 hours at 37°C at a 100:1 effector-to-target ratio with neutrophils from either WT or CR3−/− mice. Flow cytometry was performed to detect the specific cytotoxicity of CFSEhigh target cells. Percentage killing was determined by the formula [1-(ratioexperimental/ratiocontrol) x100%] where ratio = % CFSEhigh/CSFElow.
Statistical analysis
Data were entered into Prism 4.0 (GraphPad Software) to generate graphs of the percentage of fluorescent-positive cells or tumor regression, and Student t test was used to determine the significance of differences between 2 datasets. Survival curves were created using the Kaplan-Meier method and statistical analyses of survival curves utilized a log-rank test.
Results
Dectin-1 signals differentiate β-glucan phagocytosis and binding in DCs and macrophages
The dectin-1 receptor has been previously demonstrated to recognize β-glucans in zymosan30 and the cell walls of various live pathogenic fungi and is a critical phagocytic receptor for fungal internalization.31 CR3 (CD11b/CD18) has also been implicated to involve in β-glucan binding and recognition.18,32 Thus we first examined β-glucan phagocytosis by BMDCs and thioglycollate-elicited macrophages (Figure 1A-B). Particulate β-glucan is a purified S cerevisiae β-glucan particle. Soluble PGG β-glucan is a pharmaceutical-grade β-glucan with an average molecular mass of 150 kD compound derived from the same strain of yeast as particulate β-glucan. We observed that both DCs (Figure 1A) and macrophages (Figure 1B) from WT mice were capable of phagocytosing particular β-glucan. Further flow cytometry and ImageStream analysis revealed that phagocytosis of particulate β-glucan by DCs was partially inhibited in dectin-1–deficient mice but was not significantly altered in CD11b-deficient mice (Figure 1A). However, macrophage-mediated particulate β-glucan phagocytosis was completely abrogated in dectin-1-deficient mice and was not affected in CD11b-deficient mice (Figure 1B), suggesting differential use of dectin-1 by phagocytosis in macrophages and DCs. We further examined β-glucan binding activity on DCs and macrophages. Particulate β-glucan bound to DCs and macrophages was also partially dependent on dectin-1 but not the CD11b receptor (data not shown). In contrast, soluble PGG β-glucan bound to DCs and macrophages independent of dectin-1 or CD11b receptors (Figure 1C). However, soluble β-glucan PGG, but not dextran, significantly inhibited particulate β-glucan binding by both DCs and macrophages (Figure 1D).
β-glucan phagocytosis and binding on DCs and macrophages. BMDCs (A) and macrophages (B) were incubated with DTAF-WGP at 37°C and phagocytosis of particulate β-glucan was assessed by flow cytometry and ImageStream. Percent of particulate β-glucan positive cells were summarized. One representative dot plot from 3 independent experiments with similar results. (C) BMDCs and macrophages were incubated with PGG-DTAF β-glucan on ice and β-glucan binding was assessed by flow cytometry. The data indicate that soluble β-glucan binding on DCs and macrophages is independent of dectin-1 or CD11b receptors. (D) BMDCs and macrophages were incubated with soluble PGG β-glucan or dextran and then mixed with DTAF-WGP on ice. Particulate β-glucan binding on DCs or macrophages was measured by flow cytometry. Cells were gated on CD11c+ or F4/80+ cells.
β-glucan phagocytosis and binding on DCs and macrophages. BMDCs (A) and macrophages (B) were incubated with DTAF-WGP at 37°C and phagocytosis of particulate β-glucan was assessed by flow cytometry and ImageStream. Percent of particulate β-glucan positive cells were summarized. One representative dot plot from 3 independent experiments with similar results. (C) BMDCs and macrophages were incubated with PGG-DTAF β-glucan on ice and β-glucan binding was assessed by flow cytometry. The data indicate that soluble β-glucan binding on DCs and macrophages is independent of dectin-1 or CD11b receptors. (D) BMDCs and macrophages were incubated with soluble PGG β-glucan or dextran and then mixed with DTAF-WGP on ice. Particulate β-glucan binding on DCs or macrophages was measured by flow cytometry. Cells were gated on CD11c+ or F4/80+ cells.
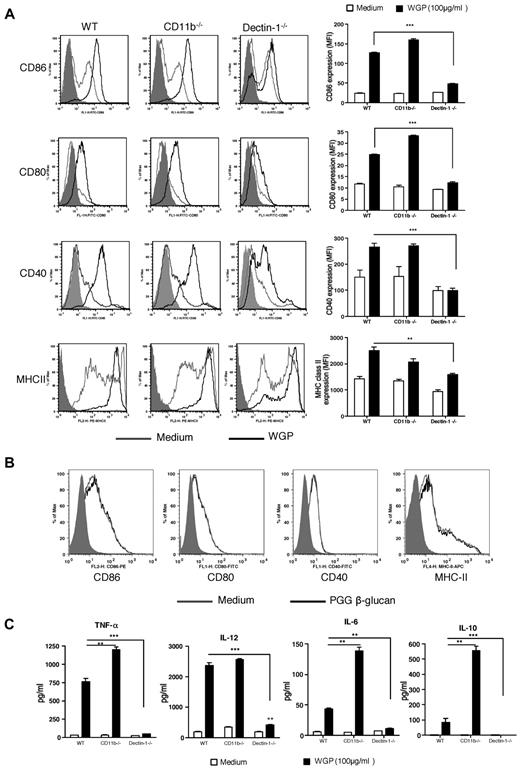
Particulate β-glucan, but not soluble β-glucan, activates DCs via a dectin-1 pathway
Next, we determined whether different preparations of β-glucans activate DCs for surface marker expression and cytokine release. DCs from WT mice stimulated with particulate β-glucan up-regulated surface markers including CD86, CD80, CD40, and major histocompatibility complex class II (Figure 2A). The expression levels of surface markers were significantly decreased in dectin-1–deficient DCs. Deficiency of CD11b did not significantly alter the particulate β-glucan–mediated effect. In contrast, soluble β-glucan did not stimulate DCs for up-regulation of surface markers (Figure 2B). We also examined cytokine levels of DCs stimulated by β-glucans. Particulate β-glucan, but not soluble β-glucan, stimulated DCs to produce large amounts of TNF-α and IL-12 but low amounts of IL-6 and IL-10 (Figure 2C and data not shown). These effects were dependent on the dectin-1 pathway. Deficiency of CD11b in DCs did not significantly alter IL-12 production but significantly increased TNF-α, IL-6 and IL-10 production (Figure 2C). Taken together, these data suggest that the up-regulation of surface markers and cytokine production in DCs stimulated by particulate β-glucan are dependent on the dectin-1 pathway and soluble β-glucan is not capable of activating DCs.
Surface marker expression and cytokine secretion by DCs stimulated with β-glucans. (A) BMDCs from WT, CD11b−/−, or dectin-1−/− mice were stimulated with particulate β-glucan (100 μg/mL) and surface marker expression was assessed by flow cytometry. One representative histogram from 3 independent experiments with similar results. (B) BMDCs were stimulated with soluble PGG β-glucan and surface marker expression was measured by flow cytometry. (C) Supernatants from particulate β-glucan–stimulated DCs were collected and assayed for TNF-α, IL-12, IL-6, and IL-10 (**P < .01; ***P < .001).
Surface marker expression and cytokine secretion by DCs stimulated with β-glucans. (A) BMDCs from WT, CD11b−/−, or dectin-1−/− mice were stimulated with particulate β-glucan (100 μg/mL) and surface marker expression was assessed by flow cytometry. One representative histogram from 3 independent experiments with similar results. (B) BMDCs were stimulated with soluble PGG β-glucan and surface marker expression was measured by flow cytometry. (C) Supernatants from particulate β-glucan–stimulated DCs were collected and assayed for TNF-α, IL-12, IL-6, and IL-10 (**P < .01; ***P < .001).
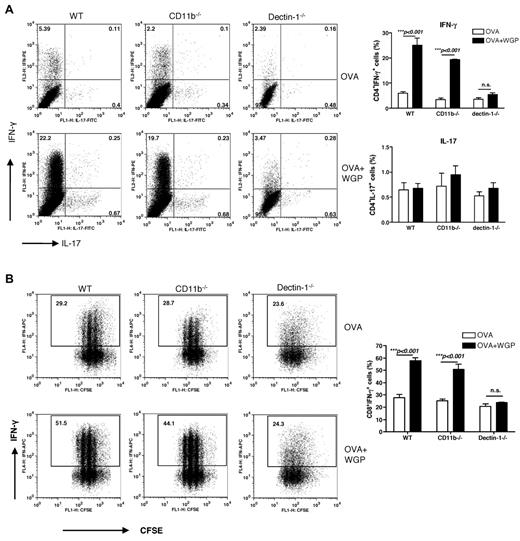
Particulate β-glucan, but not soluble β-glucan, induces enhanced Th1 and cytotoxic T lymphocyte priming in vitro
To evaluate the potential of β-glucan–activated DCs to prime CD4+ T cells, we cultured ovalbumin-specific naive CD4+ T cells with BMDCs. Addition of ovalbumin protein to the culture stimulated predominant Th1 priming and marginal Th17 differentiation. Further addition of particulate β-glucan significantly enhanced Th1 priming. The enhanced Th1 priming was dependent on the dectin-1 pathway but was independent of the CD11b receptor (Figure 3A). Th17 priming was not significantly enhanced by particulate β-glucan stimulation. Not surprisingly, only particulate β-glucan but not soluble β-glucan promoted Th1 priming (data not shown).
CD4 T-cell differentiation and CD8 priming and differentiation stimulated by β-glucan–activated DCs. (A) OVA Tg CD4+ T cells were cocultured with BMDCs from WT, CD11b−/− or dectin-1−/− mice in the presence or absence of OVA and β-glucans. CD4+ T cells cultured with β-glucans were used as controls. Percent of CD4+IFN-γ+ cells and CD4+ IL-17+ cells is shown (n = 4). (B) CFSE-labeled OVA Tg CD8+ T cells were cultured together with BMDCs in the presence of OVA and β-glucan. CD8+ T cells cultured with β-glucans were used as controls. Graphs show CFSE dilution versus intracellular IFN-γ on day 3 of culture. Percent of CD8+IFN-γ+ cells is shown (n = 4).
CD4 T-cell differentiation and CD8 priming and differentiation stimulated by β-glucan–activated DCs. (A) OVA Tg CD4+ T cells were cocultured with BMDCs from WT, CD11b−/− or dectin-1−/− mice in the presence or absence of OVA and β-glucans. CD4+ T cells cultured with β-glucans were used as controls. Percent of CD4+IFN-γ+ cells and CD4+ IL-17+ cells is shown (n = 4). (B) CFSE-labeled OVA Tg CD8+ T cells were cultured together with BMDCs in the presence of OVA and β-glucan. CD8+ T cells cultured with β-glucans were used as controls. Graphs show CFSE dilution versus intracellular IFN-γ on day 3 of culture. Percent of CD8+IFN-γ+ cells is shown (n = 4).
We next determined the potential of β-glucan–activated DCs to prime CD8+ T cells. As shown in Figure 3B, Ag ovalbumin alone was sufficient to induce OT-I T-cell proliferation as assessed by CFSE dilution assay. Further addition of particulate β-glucan but not soluble β-glucan increased CD8 T-cell proliferation (Figure 3B and data not shown). In addition, the presence of particulate β-glucan drastically increased Ag-specific CD8 T-cell differentiation into effector cells as measured by their INF-γ production. The adjuvant effect of particulate β-glucan was mediated by DCs and not T cells because the T cells did not proliferate when they were stimulated with particulate β-glucan alone (data not shown). In addition, it was dependent on the dectin-1 receptor pathway, as demonstrated by the decreased CD8 T-cell priming in culture with dectin-1–deficient BMDCs. In summary, activation of the dectin-1 pathway by particulate β-glucan rendered BMDCs fully capable of promoting naive CD4+ T-cell differentiation into Th1 and supporting expansion of naive CD8 T cells and differentiation into effector CTLs in vitro.
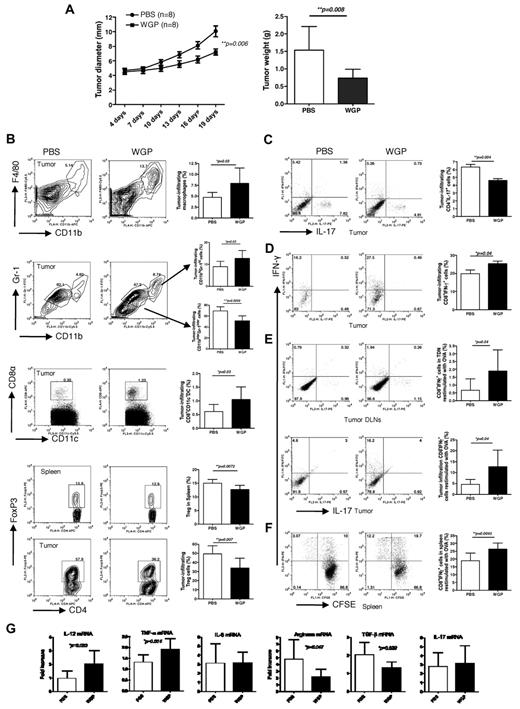
Orally administrated particulate β-glucan treatment promotes antitumor immunity via a dectin-1–dependent pathway
To determine the in vivo effect of particulate β-glucan on tumor therapy, we challenged mice with ovalbumin-transfected murine mammary carcinoma EO771 (OVA/EO771). When the tumors were palpable, mice were treated with or without orally administered particulate β-glucan daily. Tumor-bearing mice treated with particulate β-glucan had a significantly decreased tumor burden compared with the mice treated with PBS (Figure 4A). We further examined tumor-infiltrating cells in these mice. We found that particulate β-glucan treatment significantly increased macrophage and CD8α+CD11c+DC infiltration and the frequency of CD11bhiGr-1hi granulocytes within the tumor microenvironment (Figure 4B). The frequency of CD11binterGr-1inter myeloid cells in tumor and CD4+Foxp3+ Treg cells both in tumor and spleen were significantly decreased in particulate β-glucan–treated mice (Figure 4B). Particulate β-glucan treatment also significantly decreased Th17 differentiation and promoted CD8+ IFN-γ–producing T cells in the tumor milieu (Figure 4C-D). To further determine Ag-specific T-cell responses, cells from tumor DLNs, tumor mass, and spleen were restimulated with OVA. Particulate β-glucan treatment significantly increased OVA-specific CD8 T-cell expansion and further differentiation into IFN-γ–producing effector cells (Figure 4E-F).
Particulate β-glucan treatment significantly reduces tumor burden with enhanced antitumor immunity. (A) Groups of WT mice were implanted subcutaneously with EO771/OVA tumor cells. After palpable tumors formed, mice were treated daily with or without particulate β-glucan for 2 weeks. Tumor diameter was recorded at the indicated time. Tumor mass was weighted when mice were killed. (B) Single cell suspensions prepared from tumor samples or spleen as indicated were stained with fluorochrome labeled mAbs. Summarized data are shown. (C-D) Single cell suspensions from tumors were stimulated with PMA plus ionomycin and stained intracellular IFN-γ and IL-17. Cells were gated on CD4+ (C) or CD8+ (D) T cells. (E) Single cell suspensions from tumors or tumor DLNs were stimulated with ovalbumin and intracellular IFN-γ staining was performed. (F) Splenocytes were labeled with CFSE and then stimulated with ovalbumin. Graphs show CFSE dilution versus intracellular IFN-γ production. (G). RNAs from tumor specimens were extracted and qRT-PCR was performed for the indicated cytokines and arginase.
Particulate β-glucan treatment significantly reduces tumor burden with enhanced antitumor immunity. (A) Groups of WT mice were implanted subcutaneously with EO771/OVA tumor cells. After palpable tumors formed, mice were treated daily with or without particulate β-glucan for 2 weeks. Tumor diameter was recorded at the indicated time. Tumor mass was weighted when mice were killed. (B) Single cell suspensions prepared from tumor samples or spleen as indicated were stained with fluorochrome labeled mAbs. Summarized data are shown. (C-D) Single cell suspensions from tumors were stimulated with PMA plus ionomycin and stained intracellular IFN-γ and IL-17. Cells were gated on CD4+ (C) or CD8+ (D) T cells. (E) Single cell suspensions from tumors or tumor DLNs were stimulated with ovalbumin and intracellular IFN-γ staining was performed. (F) Splenocytes were labeled with CFSE and then stimulated with ovalbumin. Graphs show CFSE dilution versus intracellular IFN-γ production. (G). RNAs from tumor specimens were extracted and qRT-PCR was performed for the indicated cytokines and arginase.
Next, we examined whether cytokine profiles and arginase levels in the tumor milieu would be altered on particulate β-glucan treatment. To this end, tumors from particulate β-glucan–treated or untreated mice were excised and RNAs were extracted for real-time PCR. As shown in Figure 4G, the mRNA levels of IL-6 and IL-17 were not significantly changed. Strikingly, IL-12 and TNF-α production were significantly increased while the mRNA levels of arginase and transforming growth factor-β were significantly decreased within the tumor microenvironment after particulate β-glucan treatment. These data suggest that particulate β-glucan treatment is capable of modulating the suppressive tumor microenvironment and promoting Th1 and cytotoxic T lymphocyte responses, leading to the decreased tumor burden.
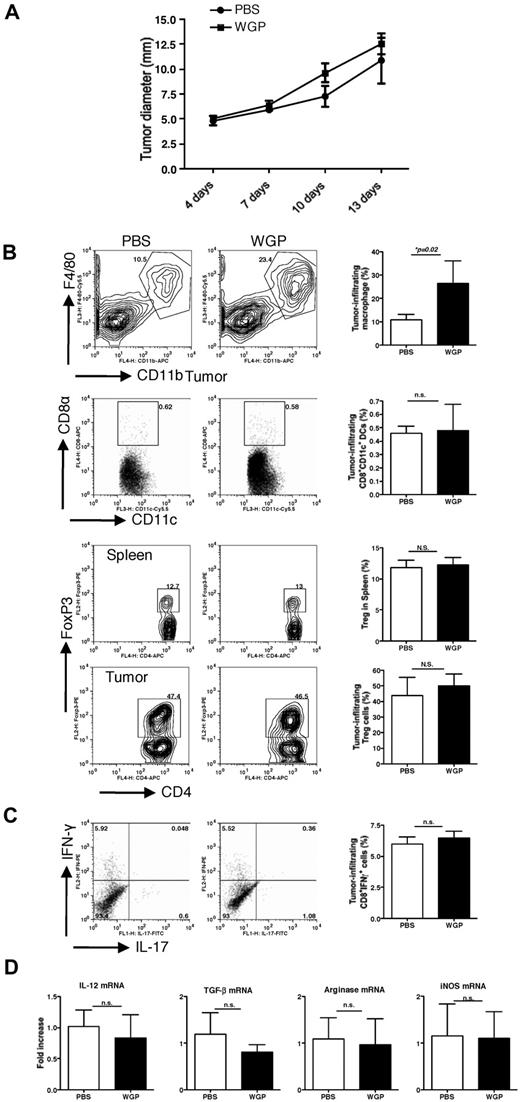
Having demonstrated the importance of the dectin-1 pathway in particulate β-glucan–mediated in vitro effect, the same tumor protocol was performed in dectin-1–deficient mice. Tumors in dectin-1–deficient mice appeared to grow faster than those in WT mice. Particulate β-glucan treatment did not significantly decrease tumor progression (Figure 5A). Further analysis of tumor-infiltrating cells revealed that particulate β-glucan treatment significantly increased macrophage infiltration within the tumor regardless of dectin-1 deficiency. However, the observed antitumor effects of particulate β-glucan in WT mice including increased CD8+CD11c+DCs in tumor, decreased Treg in spleen and tumor, and increased CD8+IFN-γ–producing T cells were completely abrogated in dectin-1–deficient mice (Figure 5B). Furthermore, the modulatory effect of particulate β-glucan on the cytokines and arginase levels were also diminished in dectin-1–deficient mice (Figure 5C). Thus, the dectin-1 receptor is essential for particulate β-glucan–mediated antitumor immune responses in vivo.
Dectin-1–deficient completely abrogates particulate β-glucan–mediated antitumor therapeutic efficacy. (A) Groups of dectin-1−/− mice were implanted subcutaneously with EO771/OVA tumor cells. After palpable tumors formed, mice were treated daily with or without particulate β-glucan for 2 weeks. Tumor diameter was recorded at the indicated time. (B) Single cell suspensions prepared from tumor samples or spleen as indicated were stained with fluorochrome labeled mAbs. Summarized data are shown. (C) Single cell suspensions from tumors were also stimulated with PMA plus ionomycin and stained intracellular IFN-γ and IL-17. Cells were gated on CD8+ T cells. (D) RNAs from tumor specimens were extracted and qRT-PCR was performed for the indicated cytokines and arginase.
Dectin-1–deficient completely abrogates particulate β-glucan–mediated antitumor therapeutic efficacy. (A) Groups of dectin-1−/− mice were implanted subcutaneously with EO771/OVA tumor cells. After palpable tumors formed, mice were treated daily with or without particulate β-glucan for 2 weeks. Tumor diameter was recorded at the indicated time. (B) Single cell suspensions prepared from tumor samples or spleen as indicated were stained with fluorochrome labeled mAbs. Summarized data are shown. (C) Single cell suspensions from tumors were also stimulated with PMA plus ionomycin and stained intracellular IFN-γ and IL-17. Cells were gated on CD8+ T cells. (D) RNAs from tumor specimens were extracted and qRT-PCR was performed for the indicated cytokines and arginase.
Soluble β-glucan augments antitumor monoclonal antibody-mediated therapeutic efficacy via complement dependent but dectin-1 independent pathways
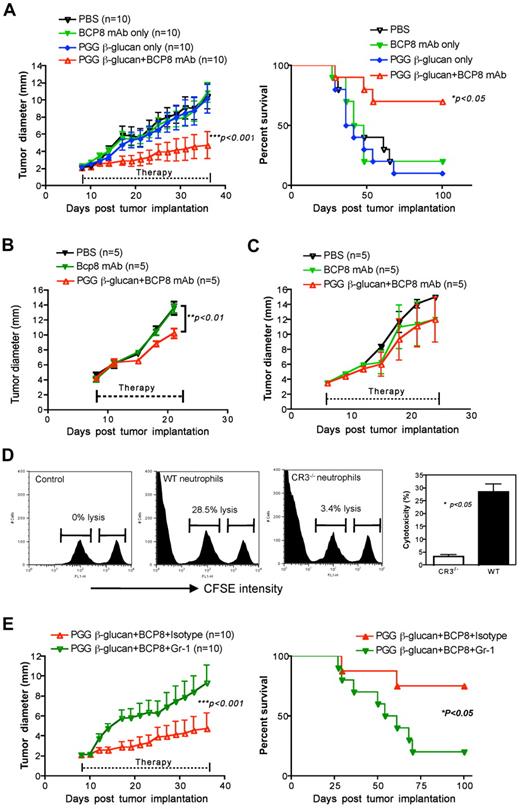
To determine the therapeutic efficacy of yeast-derived soluble β-glucan, mice were inoculated with murine lymphoma RMA-MUC1 cells. Because our previous studies have demonstrated that soluble β-glucan can induce CR3-dependent cellular cytotoxicity (CR3-DCC),33 antitumor MUC1 monoclonal antibody (BCP8) was administered to induce complement activation. As shown in Figure 6A, tumor-bearing mice treated with antitumor monoclonal antibody or PGG soluble β-glucan alone did not render tumor regression. Combined soluble β-glucan with antitumor monoclonal antibody achieved a significant decreased tumor progression (Figure 6A). Most of these mice survived at the cessation of treatment. To further explore whether the dectin-1 pathway plays any role in the combination therapy, we carried the same protocol in dectin-1–deficient mice. Similar to the EO771 tumor model, murine lymphoma tumors grew much faster than those in WT mice. Nevertheless, tumor-bearing mice treated with combined soluble β-glucan with antitumor monoclonal antibody therapy exhibited a significantly decreased tumor burden in dectin-1–deficient mice (Figure 6B). However, the efficacy was completely abrogated in C3-deficient mice, indicating that complement activation is required for the adjuvant effect of soluble β-glucan on antitumor monoclonal antibody therapy (Figure 6C). Our previous studies demonstrate that CR3+ neutrophils are critical in combined β-glucan with antitumor monoclonal antibody therapy.33-35 Thus, innate neutrophils were marginated from soluble PGG β-glucan–treated WT or CR3−/− mice and cocultured with CSFE labeled iC3b-opsonized (CFSEhigh) or un-opsonized (CFSElow) RAM-MUC1 lymphoma cells as target cells. As shown in Figure 6C, neutrophils from soluble PGG β-glucan–treated WT mice but not CR3-deficient mice were capable of killing iC3b-opsonized tumor cells, suggesting critical role for CR3 expression on neutrophils. Furthermore, therapeutic efficacy mediated by combined soluble β-glucan with antitumor monoclonal antibody was abrogated when neutrophils were depleted in treated animals (Figure 6E).
Soluble β-glucan augments antitumor monoclonal antibody-elicited therapeutic efficacy. (A) Groups of WT mice were implanted subcutaneously with RMA-MUC1, after 7 days, to allow tumor formation, were treated with different regimens. Both tumor progression and tumor-free survival were monitored. (B) Dectin-1−/− or (C) C3−/− mice were implanted with RMA-MUC1 tumor cells. After tumors were formed, mice were subject to different treatment as indicated. Tumor diameter was recorded at the indicated time. (D) Neutrophils were marginated from WT and CD11b−/− mice treated with soluble PGG β-glucan for 1 week. iC3b-opsonized tumor cells and un-opsonized tumor cells were labeled with a different intensity of CFSE and mixed at a 1:1 ratio and incubated with neutrophils. Percent of cytotoxicity is shown. (E) WT mice treated with soluble PGG β-glucan in combination with antitumor mAbs were preinjected with anti–Gr-1 monoclonal antibody to deplete neutrophils or isotype the control monoclonal antibody. Tumor diameter and tumor-free survival were monitored.
Soluble β-glucan augments antitumor monoclonal antibody-elicited therapeutic efficacy. (A) Groups of WT mice were implanted subcutaneously with RMA-MUC1, after 7 days, to allow tumor formation, were treated with different regimens. Both tumor progression and tumor-free survival were monitored. (B) Dectin-1−/− or (C) C3−/− mice were implanted with RMA-MUC1 tumor cells. After tumors were formed, mice were subject to different treatment as indicated. Tumor diameter was recorded at the indicated time. (D) Neutrophils were marginated from WT and CD11b−/− mice treated with soluble PGG β-glucan for 1 week. iC3b-opsonized tumor cells and un-opsonized tumor cells were labeled with a different intensity of CFSE and mixed at a 1:1 ratio and incubated with neutrophils. Percent of cytotoxicity is shown. (E) WT mice treated with soluble PGG β-glucan in combination with antitumor mAbs were preinjected with anti–Gr-1 monoclonal antibody to deplete neutrophils or isotype the control monoclonal antibody. Tumor diameter and tumor-free survival were monitored.
Discussion
The use of β-glucan as a complementary and alternative medicine has prevailed for centuries in traditional Asian medicine. Recent studies demonstrate that β-glucans can also function as a potent adjuvant to stimulate innate and adaptive immune responses.13,15 β-glucans from different sources appear to function differently for T-cell differentiation. For example, DCs activated by bacterial β-glucan Curdlan stimulate Th1, Th17, and cytotoxic T lymphocyte priming and differentiation,13,14 and convert Treg into IL-17–producing T cells36 via a dectin-1–dependent pathway. The yeast zymosan β-glucan appears to induce regulatory Ag-presenting cells leading to Treg differentiation.28,37 Bacterial β-glucan Curdlan is a linear β(1,3)-glucan without branching while yeast-derived β-glucan has the β(1,3) backbone branched with β(1,6)-linked side chains. In this study, we demonstrate that different preparations of yeast-derived β-glucans, particulate versus soluble, stimulate immune responses via differential pathways.
We found that particulate β-glucan was phagocytosed by both DCs and macrophages. However, phagocytosis by macrophages was completely dependent on the dectin-1 receptor while dectin-1–deficient DCs were still capable of phagocytosing particulate β-glucans. Similarly, particulate β-glucan binding was not completely blocked in dectin-1–deficient mice (data not shown). These data are consistent with a previous study suggesting that DCs and macrophages may use a differential dectin-1 pathway.38 Interestingly, soluble β-glucan bound to DCs or macrophages independent of dectin-1 or CR3 receptors. However, soluble β-glucan significantly inhibited particulate β-glucan binding by DCs and macrophages, suggesting that β-glucan recognition is essential. Although particulate β-glucan binding and phagocytosis by DCs were partially dependent on the dectin-1 receptor, activation of DCs for up-regulation of surface accessory molecules critical for stimulating adaptive T-cell responses and cytokine release was largely dependent on the dectin-1 pathway. In contrast, soluble β-glucan bound to DCs but did not activate DCs for up-regulation of surface molecules and cytokine release. Furthermore, in vitro T-cell priming analysis demonstrated that particulate β-glucan predominately stimulated Th1 responses. Th17 differentiation was not significantly altered on particulate yeast-derived β-glucan stimulation, which is different from the bacterial β-glucan Curdlan.14 Particulate yeast-derived β-glucan also stimulated CD8+ T-cell priming and differentiation into effector cells. All these effects were significantly decreased in dectin–1 deficient mice.
Orally administered particulate β-glucan could be taken up by DCs and macrophages and trafficked into lymphoid tissue.15,39 The in vivo adjuvant effect of particulate β-glucan was demonstrated in a murine tumor model. Orally administered particulate β-glucan treatment significantly delayed tumor progression, which is consistent with our previous study.15 We also observed that particulate β-glucan treatment promoted Ag-specific CD8 T-cell responses and modulated the suppressive tumor microenvironment including decreased myeloid cells and Treg, and decreased mRNA levels of transforming growth factor-β and arginase in tumors. The decreased myeloid cell accumulation could be because of the fact that particulate β-glucan promoted myeloid cells further differentiation and maturation to macrophages and DCs as we observed in vitro (data not shown). This may also contribute to the fewer Treg cells that occurred in particulate β-glucan–treated mice. Although particulate β-glucan did not stimulate Th17 differentiation in vitro, in vivo treatment of particulate β-glucan decreased Th17 cells in the tumors. This is probably because of the strong, biased Th1 responses stimulated by particulate β-glucan. The roles of IL-17 and Th17 within the tumors are still controversial depending on different tumor models.40,41 Particulate β-glucan also significantly increased CD8α+CD11c+DCs within the tumors. This subset of DCs is capable of taking up dead or dying cells and has a superior capacity to cross-present exogenous Ags on major histocompatibility complex class I molecules to CD8 T cells.42,43 Although in the setting of infection, DCs migrate to the draining lymph nodes on interaction with pathogens, where they prime T cells. The findings that significant increased CD8α+CD11c+DCs and IFN-γ–producing CD8+ T cells in tumors lead us to hypothesize that DCs might also migrate into the tumor to promote local expansion of T cells that were previously activated. This notion is supported by a recent study showing that a substantial reduction of T-cell infiltration in the kidneys of lupus-prone mice occurred in the absence of DCs.44 Thus, it is proposed that dead or dying tumor cells are taken up by CD8+CD11c+DCs. These DCs are activated by particulate β-glucan and in turn cross-present Ags to CD8 T cells for priming and differentiation. In addition, particulate β-glucan treatment significantly increased tumor-infiltrating macrophages with a high major histocompatibility complex class II expression level (data not shown), indicating an M1/activated macrophage phenotype. These macrophages may also promote Th1 response in vivo. These data reaffirm that particulate β-glucan can be used as a potent adjuvant to promote antitumor Th1 and cytotoxic T lymphocyte responses. Strikingly, all observed effects mediated by particulate β-glucan, except macrophage infiltration in the tumors, were completely abrogated in dectin-1–deficient mice. It is worth noting that tumors of both models grow much faster in dectin-1–deficient mice than those in WT mice, indicating that the dectin-1 receptor may be critical in the immunosurveillance mechanism. Indeed, human dectin-1 deficiency leads to mucocutaneous fungal infections.45 Taken together, we conclude that the dectin-1 pathway is essential for particulate β-glucan to stimulate innate and adaptive antitumor immune responses.
Compared with particulate β-glucan, soluble β-glucan did not stimulate DC activation although it bound to DCs. Not surprisingly, it did not stimulate any Th1 or cytotoxic T lymphocyte priming and differentiation in vitro. It is possible that the engagement of soluble β-glucan alone is not sufficient to induce receptor crosslinking thus triggering cytokine release. However, this is not related to the sizes of soluble β-glucans because larger MW soluble β-glucans with MW 220 kD and 400 kD did not stimulate DCs for cytokines (C.Q. et al, unpublished observations). Consistently, soluble β-glucan alone therapy did not have any therapeutic efficacy in murine lymphoma model. However, soluble β-glucan significantly promoted antitumor monoclonal antibody-mediated therapeutic efficacy as demonstrated by tumor progression and long-term tumor-free survival (Figure 6A). These effects were not dependent on the dectin-1 pathway but required a complement activation. Furthermore, CR3+ neutrophils were demonstrated to be crucial for the combined therapy, which is consistent with our previous studies.34,46 In vivo, soluble β-glucan is processed by macrophages to release a small active moiety that is capable of binding to the CR3 lectin-like domain thus priming CR3+ effectors.33 Subsequently, β-glucan–primed effector cells are able to kill iC3b-opsonized tumor cells mediated by complement-activating antitumor mAbs. Dual ligation of CR3 by soluble β-glucan and iC3b leads to degranulation and cytotoxic effects of CR3+ neutrophils. Thus soluble β-glucan primes innate neutrophils and synergizes with complement-activating antitumor mAbs to engage CR3-DCC for potentiating its therapeutic efficacy.
Why do particulate β-glucan and soluble β-glucan use differential receptor pathways for their biologic effect? In the current study, particulate and soluble β-glucans are both purified from the same yeast strain S cerevisiae. Particulate β-glucan is a yeast cell wall preparation and is prepared by subjecting the yeast cells to a series of alkaline, surfactant, and acidic extractions that remove host cell impurities. The cell wall of S cerevisiae is predominantly composed of linear helical chains of β-D-glucose molecules bonded through 1,3 linkages. These β-1,3-glucan chains also contain a number of 1,6 branch points. Soluble PGG β-glucan is extracted from particulate β-glucan through the hydrosol solubilization, clarification, and further purification using hydrophobic interaction chromatograph (HIC) and gel permeation chromatograph. Previous studies demonstrated that soluble PGG β-glucan is composed of triple helices and aggregates as triplets in solution.47 Crystal structure analysis of the dectin-1 protein indicates that a shallow groove running between Trp221 and His223 in dectin-1 is a critical β-glucan–binding domain.48,49 It is possible that the conformation of triple helices in soluble PGG β-glucan may influence the dectin-1 binding site. In addition, structural variables including (1,3)-β–linked backbone structure, polymer length, and side-chain branching are also critical for β-glucan–binding on the dectin-1 receptor.50
In summary, we demonstrate that particulate β-glucan is capable of stimulating innate and adaptive immune responses via a dectin-1 pathway while soluble β-glucan primes innate neutrophils for tumoricidal activity via complement and CR3-dependent pathways. These data highlight the differential ability of different preparations of β-glucans in the adjuvant therapy and allow for the rational design of immunotherapeutic protocols usable in clinical trials.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by research funding from National Institutes of Health R01 CA86412, R01 CA150947 and the Kentucky Lung Cancer Research Board (J.Y.), and Natural Science Foundation of China 30901304 (C.Q.).
National Institutes of Health
Authorship
Contribution: C.Q. and Y.C. helped design experiments, performed experiments, analyzed data, and wrote the paper; L.G., C.D., and B.L. performed supporting experiments; G.K., K.Q., J.V., and J.R.Y., helped design experiments; S.S., and Y.I., developed and provided dectin-1–deficient mice; and J.Y. designed research, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: J.Y. and J.V. have declared a financial interest in Biothera whose products were studied in the present work. The remaining authors declare no competing financial interests.
Correspondence: Jun Yan, MD, PhD, Tumor Immunobiology Program, James Graham Brown Cancer Center, Clinical & Translational Research Bldg, Rm 319, University of Louisville, 505 S Hancock St, Louisville, KY 40202; e-mail: jun.yan@louisville.edu.
References
Author notes
C.Q. and Y.C. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal