Abstract
POEMS is an uncommon syndromic disorder characterized by polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes. There are few descriptions of the bone marrow pathology of POEMS; therefore, peripheral blood smears and bone marrow aspirates and biopsies from 87 patients (143 total, 67 pretreatment, 76 posttreatment cases) with POEMS were studied. Plasma cell clonality was analyzed by flow cytometry, immunohistochemistry, and/or in situ hybridization. Monotypic plasma cells were detected in 44 pretreatment cases (66%); the majority of plasma cells expressed λ light chain (91%). The monotypic plasma cells typically were present in a background of increased polytypic plasma cells. Lymphoid aggregates were found in 33 (49%) pretreatment cases and in most cases were rimmed by plasma cells (97%). Megakaryocyte hyperplasia (36 cases) and clusters (62 cases) were frequent; however, none of the 43 cases tested had the JAK2V617F mutation. In summary, we have identified a novel constellation of features that should strongly suggest POEMS syndrome as part of the differential diagnosis. The constellation of λ-restricted monoclonal gammopathy, plasma cell rimming around lymphoid aggregates, and megakaryocytic hyperplasia in a bone marrow is highly suggestive of this diagnosis, especially in the context of a peripheral neuropathy.
Introduction
In 1980, Bardwick and colleagues1 first proposed the acronym “POEMS syndrome” to describe those patients who present with polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes. The syndrome has also been referred as osteosclerotic myeloma, Crow-Fukase syndrome, and Takatsuki syndrome.2,3 Other features of this syndrome that may be present include sclerotic bone lesions, Castleman disease (CD), extravascular volume overload (peripheral edema, pleural effusion, and ascites), papilledema, clubbing, and pulmonary disease. Laboratory findings in patients with POEMS syndrome often include thrombocytosis, erythrocytosis, and elevated levels of vascular endothelial growth factor (VEGF).3-5
The diagnosis of POEMS syndrome is often difficult to establish because not all features that are part of the acronym are present in all patients. However, polyneuropathy and a monoclonal plasma cell proliferative disorder (PCPD) are by definition present in all patients,5 with symptoms related to the peripheral neuropathy often leading the patient to seek medical attention. Given the rarity of the condition, these patients frequently are misdiagnosed as having a chronic demyelinating polyneuropathy, or when the monoclonal protein is subsequently recognized, primary amyloidosis (AL type). A monoclonal gammopathy of undetermined significance (MGUS)–related peripheral neuropathy also may be considered.
The histopathologic findings that correspond to the presenting clinical features are, at best, poorly characterized. Between 11% and 30% of patients with POEMS will have coexistent CD or lymph node pathology findings that are often interpreted as “Castleman-like.”3,6 Approximately one-third of patients will have localized BM plasmacytoma(s) without widespread BM involvement. Even this latter group without widespread BM involvement will typically have < 10% plasma cells (PCs) on iliac crest biopsy.3,4,6 Apart from studies of the marrow PC burden in POEMS, there are no other descriptions of the disorder-associated BM histology. Recognition of these features is critically important given the challenges in clinically recognizing affected patients. For this reason, the BM pathology in a large group of POEMS patients was evaluated in this study and compared with control groups of PCPD, lymphoplasmacytic lymphoma (LPL), and normal BM.
Methods
Patients
We queried the Mayo Clinic dysproteinemia database from 1996 to 2009 for “POEMS” and identified 87 patients from Mayo Clinic in Rochester, Minnesota, who had a clinical diagnosis of POEMS syndrome and BM aspirate/biopsies performed at Mayo Clinic. One patient had atypical POEMS (no polyneuropathy but had other characteristics of the syndrome, including monotypic λ-restricted PCs, hepatosplenomegaly, edema, elevated VEGF levels, severe papilledema, and thrombocytosis) and was included in the study. A total of 143 BM examinations with peripheral smears were available on these patients between August 1996 and January 2009; 67 were obtained before treatment and 76 after therapy (alkylator-based chemotherapy, radiation, and or peripheral blood stem cell transplant). Complete clinical histories and laboratory data were available for all patients. The study was approved by the Mayo Clinic Institutional Review Board.
Light microscopic analysis
All BM studies included review of the peripheral blood smear, BM aspirate smear, and unilateral BM trephine biopsy. Peripheral blood and BM aspirate smears were stained by Wright-Giemsa per standard methods. BM biopsies were decalcified by the use of a 20% formic acid solution, fixed with B5, embedded in paraffin, and stained with hematoxylin and eosin per standard methods. A subset of the BM biopsies also were stained with a Gomori reticulin stain (n = 138) or a Congo red stain (n = 50) for amyloid with the use of standard methodologies. In addition, a series of BM biopsies from non-POEMS patients were studied as controls and consisted of 20 normal BMs, 47 PCPD (involved by 20% or less monotypic PCs), and 35 LPL cases (part of a previous study by our group7 ).
Immunophenotyping
PC clonality was determined in the BM aspirate specimens in all cases. Because these patients were studied during an extended period of time, various methods were used to confirm PC clonality, including a manual fluorescent analysis or a 2-color, 3-color, 4-color (n = 102), or (currently) 6-color (n = 41) flow cytometric (FC) analysis with the use of described methodologies.7,8 Immunohistochemical (IHC) studies (streptavidin-biotin peroxidase complex method) used antibodies directed against the following: CD3 (n = 15, clone PS1; Leica Novacastra), CD20 (n = 17, clone L26; Dako), CD138 (n = 141, clone MI15; Dako), HHV8 (n = 32, clone 13B10; Leica Novacastra), and κ and λ immunoglobulin light chains (n = 138, rabbit polyclonal; Dako). In situ hybridization (ISH) studies were performed with the use of probes that recognize κ and λ immunoglobulin light chains (n = 5; Ventana).
Mutational analysis
Because these patients may present with elevated platelet or erythrocyte counts, molecular testing for the JAK2V617F mutation was performed on archived frozen cell suspensions or performed as part of clinical care in a subset of cases (n = 43) by the use of an allele-specific PCR method.9
Results
The clinical and pathologic findings are summarized in Tables 1 and 2, respectively. An M-spike was detected by serum protein electrophoresis and/or immunofixation in 83 of 87 total patients. Of the 4 patients who did not have an M-spike, one patient had a λ-restricted plasmacytoma at a distant site, one patient had λ-restricted PCs in the BM biopsy, one patient had monotypic λPCs in a lymph node biopsy diagnostic of CD, and one patient had an M-spike (λ) detected at another institution. The peripheral blood smears showed morphologically unremarkable red blood cells, white blood cells, and platelets.
Summary of clinical findings of patients with POEMS syndrome
| . | Total patients, n = 87 . |
|---|---|
| Age, y (range, median) | (20-74, 49) |
| Sex | |
| Male | 57 |
| Female | 30 |
| CBC | |
| Hemoglobin, g/dL (range, median) | (7.8-17.7, 13.7) |
| White blood cells, ×109/L (range, median) | (0.3-18.8, 6.4) |
| Platelets, ×109/L (range, median) | (21-1281, 371) |
| Serum protein studies | |
| IgA λ | 39 |
| IgG λ | 32 |
| IgM λ | 1 |
| IgA λ and IgG λ | 2 |
| IgG κ and IgA λ | 5 |
| IgG κ and IgG λ | 1 |
| IgG κ | 2 |
| IgA κ and IgGκ | 1 |
| None detected | 4 |
| Polyneuropathy | 86 |
| Organomegaly* | |
| Hepatomegaly | 26 |
| Splenomegaly | 45 |
| Lymphadenopathy | 43 |
| None | 23 |
| Endocrinopathy | 78 |
| Skin changes | 71 |
| CD | 10 |
| Plasma cell variant CD | 4 |
| Hyaline vascular variant CD | 2 |
| Mixed CD | 2 |
| Unspecified CD | 2 |
| CD-like changes | 5 |
| Edema | 75 |
| Papilledema | 24 |
| Sclerotic bone lesions | 73 |
| . | Total patients, n = 87 . |
|---|---|
| Age, y (range, median) | (20-74, 49) |
| Sex | |
| Male | 57 |
| Female | 30 |
| CBC | |
| Hemoglobin, g/dL (range, median) | (7.8-17.7, 13.7) |
| White blood cells, ×109/L (range, median) | (0.3-18.8, 6.4) |
| Platelets, ×109/L (range, median) | (21-1281, 371) |
| Serum protein studies | |
| IgA λ | 39 |
| IgG λ | 32 |
| IgM λ | 1 |
| IgA λ and IgG λ | 2 |
| IgG κ and IgA λ | 5 |
| IgG κ and IgG λ | 1 |
| IgG κ | 2 |
| IgA κ and IgGκ | 1 |
| None detected | 4 |
| Polyneuropathy | 86 |
| Organomegaly* | |
| Hepatomegaly | 26 |
| Splenomegaly | 45 |
| Lymphadenopathy | 43 |
| None | 23 |
| Endocrinopathy | 78 |
| Skin changes | 71 |
| CD | 10 |
| Plasma cell variant CD | 4 |
| Hyaline vascular variant CD | 2 |
| Mixed CD | 2 |
| Unspecified CD | 2 |
| CD-like changes | 5 |
| Edema | 75 |
| Papilledema | 24 |
| Sclerotic bone lesions | 73 |
Values are numbers unless otherwise indicated.
CBC indicates complete blood count; CD, Castleman disease; and POEMS, polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes.
Patients exhibited one or more of these findings
Summary of pathologic findings of patients with POEMS syndrome
| . | Total cases (n = 143) . | Pretreatment cases (n = 67) . | Posttreatment cases (n = 76) . |
|---|---|---|---|
| Peripheral blood smear | |||
| Erythrocytosis (Hgb > 17.5 g/dL male, > 15.5 g/dL female) | 5 | 5 | 0 |
| Leukocytosis (WBC > 10.5 × 109/L) | 19 | 14 | 5 |
| Thrombocytosis (> 450 × 109/L) | 45 | 31 | 14 |
| Circulating PCs | 0 | 0 | 0 |
| Dysplastic changes | 0 | 0 | 0 |
| BM aspirate and biopsy findings | |||
| RBC atypia | 0 | 0 | 0 |
| Granulocytic atypia | 0 | 0 | 0 |
| BM cellularity | |||
| Normocellular | 85 | 43 | 42 |
| Hypercellular | 22 | 16 | 6 |
| Hypocellular | 36 | 8 | 28 |
| Lymphoid aggregate | 59 | 33 | 26 |
| PC rimming | 54/59 | 32/33 | 22/26 |
| Megakaryocyte (meg) | |||
| Cytologic atypia | 40 | 29 | 11 |
| Clusters present | 112 | 62 | 50 |
| > 5 clusters | 34/112 | 25/62 | 9/50 |
| Megs/hpf, range | 0.2-20.7 (median, 4.8) | (2.8-20.7, 6.3) | (0.2-11.5, 3.1) |
| Meg hyperplasia | 50 | 36 | 14 |
| Plasma cells | |||
| % PCs, range | < 5-60 (median, < 5) | < 5-60 (median, < 5) | < 5-30 (median, < 5) |
| PC atypia (binucleated, nucleoli, Russell or Dutcher bodies, large size) | 41 | 27 | 14 |
| Interstitial PC and perivascular | 36 | 6 | 30 |
| Small clusters | 64 | 34 | 30 |
| Large clusters | 43 | 27 | 16 |
| Monotypic λ | 72 | 40 | 32 |
| Monotypic κ | 4* | 4† | 0 |
| Polytypic light chain | 67 | 23 | 44 |
| Amyloid | 0/49 | 0/33 | 0/16 |
| HHV8 | 0/32 | 0/20 | 0/12 |
| JAK2V617F | 0/43 | NA | NA |
| . | Total cases (n = 143) . | Pretreatment cases (n = 67) . | Posttreatment cases (n = 76) . |
|---|---|---|---|
| Peripheral blood smear | |||
| Erythrocytosis (Hgb > 17.5 g/dL male, > 15.5 g/dL female) | 5 | 5 | 0 |
| Leukocytosis (WBC > 10.5 × 109/L) | 19 | 14 | 5 |
| Thrombocytosis (> 450 × 109/L) | 45 | 31 | 14 |
| Circulating PCs | 0 | 0 | 0 |
| Dysplastic changes | 0 | 0 | 0 |
| BM aspirate and biopsy findings | |||
| RBC atypia | 0 | 0 | 0 |
| Granulocytic atypia | 0 | 0 | 0 |
| BM cellularity | |||
| Normocellular | 85 | 43 | 42 |
| Hypercellular | 22 | 16 | 6 |
| Hypocellular | 36 | 8 | 28 |
| Lymphoid aggregate | 59 | 33 | 26 |
| PC rimming | 54/59 | 32/33 | 22/26 |
| Megakaryocyte (meg) | |||
| Cytologic atypia | 40 | 29 | 11 |
| Clusters present | 112 | 62 | 50 |
| > 5 clusters | 34/112 | 25/62 | 9/50 |
| Megs/hpf, range | 0.2-20.7 (median, 4.8) | (2.8-20.7, 6.3) | (0.2-11.5, 3.1) |
| Meg hyperplasia | 50 | 36 | 14 |
| Plasma cells | |||
| % PCs, range | < 5-60 (median, < 5) | < 5-60 (median, < 5) | < 5-30 (median, < 5) |
| PC atypia (binucleated, nucleoli, Russell or Dutcher bodies, large size) | 41 | 27 | 14 |
| Interstitial PC and perivascular | 36 | 6 | 30 |
| Small clusters | 64 | 34 | 30 |
| Large clusters | 43 | 27 | 16 |
| Monotypic λ | 72 | 40 | 32 |
| Monotypic κ | 4* | 4† | 0 |
| Polytypic light chain | 67 | 23 | 44 |
| Amyloid | 0/49 | 0/33 | 0/16 |
| HHV8 | 0/32 | 0/20 | 0/12 |
| JAK2V617F | 0/43 | NA | NA |
Values are numbers unless otherwise indicated.
Hgb indicates hemoglobin; HHV-8, human herpes virus-8; hpf, high power field; NA, not available; PC, plasma cell; POEMS, polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes; RBC, red blood cell; and WBC, white blood cell.
One case with biclonal plasma cell populations: IgA λ-POEMS and IgG κ-multiple myeloma.
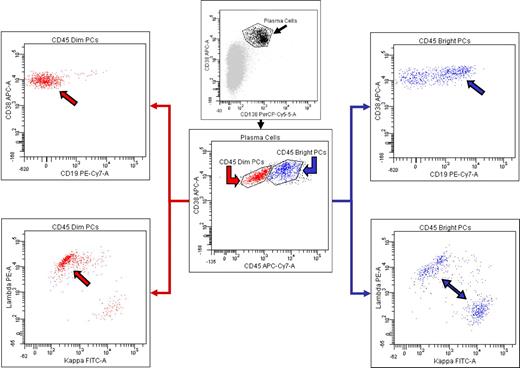
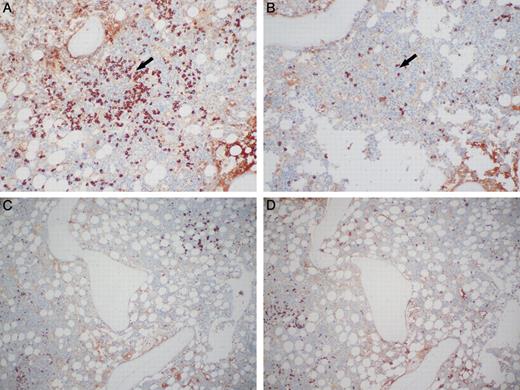
We will discuss the BM findings in pretreatment patients first. The percentage of PCs identified in the BM specimens ranged from < 5% to 60% with a median of < 5% in the 67 pretreatment BMs. In 44 of 67 (66%) pretreatment BMs, a monoclonal PC population could be detected by one or more of the methodologies used. No clonal PCs were detected in the iliac crest biopsy in the other 23 cases; all contained 5% or less PCs. Ninety-one percent of specimens with clonal PCs were λ light chain restricted. In the 4 κ-restricted cases, one patient was biclonal by serum protein immunofixation (IgA λ and IgG κ) and had λ-restricted PCs in a localized plasmacytoma and κ-restricted PCs in the BM. Twenty-five BMs had clonal PCs identified by FC studies. Seventeen of the 67 pretreatment cases were analyzed by 6-color FC analysis, and a monoclonal PC population was identified in 7 cases; the monoclonal PCs were identified within a background of polyclonal PCs (Figure 1). In 19 of the 44 pretreatment cases with monotypic PCs, FC studies showed a polyclonal PC population, but IHC (n = 18) and/or ISH (n = 1) for κ and λ light chains on the BM biopsy showed monotypic PCs. Most typically, the morphologic distribution of the PCs cells was focal aggregates of λ-restricted PCs in a background of increased polyclonal PCs (Figure 2).
Six-color FC reveals the presence of both abnormal, λ light chain-restricted PCs, and normal, polytypic PCs. Gating on the PCs brightly positive for CD38 and CD138 (upper middle, black arrow) reveals the presence of 2 distinct PC populations as determined by differential expression of CD45 (lower middle). Selective analysis of the PCs with diminished CD45 expression (lower middle, red arrow) reveals that they have abnormal loss of CD19 expression (upper left, red arrow) and have a monotypic staining pattern for λ cytoplasmic immunoglobulin light chains (lower left, red arrow). In contrast, the PCs with relatively brighter CD45 expression (lower middle, blue arrow) express CD19 (upper right, blue arrow) and show polytypic cytoplasmic immunoglobulin light chain expression (lower right, blue arrows).
Six-color FC reveals the presence of both abnormal, λ light chain-restricted PCs, and normal, polytypic PCs. Gating on the PCs brightly positive for CD38 and CD138 (upper middle, black arrow) reveals the presence of 2 distinct PC populations as determined by differential expression of CD45 (lower middle). Selective analysis of the PCs with diminished CD45 expression (lower middle, red arrow) reveals that they have abnormal loss of CD19 expression (upper left, red arrow) and have a monotypic staining pattern for λ cytoplasmic immunoglobulin light chains (lower left, red arrow). In contrast, the PCs with relatively brighter CD45 expression (lower middle, blue arrow) express CD19 (upper right, blue arrow) and show polytypic cytoplasmic immunoglobulin light chain expression (lower right, blue arrows).
κ and λ light chain immunostaining of monotypic λ PCs in a polytypic background. (A) Lymphoid aggregate (arrow) rimmed by PCs that are monotypic for λ immunoglobulin light chain. (B) PCs are negative for κ immunoglobulin light chains around the lymphoid aggregate (arrow). (C) The same case as panels A and B showing background PCs that are polytypic for immunoglobulin lights chains, λ, and (D) κ. Photomicrographic images were obtained with an Olympus BX51 microscope equipped with an Olympus DP71 camera and software. Final image preparation was performed with Adobe Photoshop CS2 for panels A and B. Original magnification 10×/0.30 UPlanFL N lens for all panels.
κ and λ light chain immunostaining of monotypic λ PCs in a polytypic background. (A) Lymphoid aggregate (arrow) rimmed by PCs that are monotypic for λ immunoglobulin light chain. (B) PCs are negative for κ immunoglobulin light chains around the lymphoid aggregate (arrow). (C) The same case as panels A and B showing background PCs that are polytypic for immunoglobulin lights chains, λ, and (D) κ. Photomicrographic images were obtained with an Olympus BX51 microscope equipped with an Olympus DP71 camera and software. Final image preparation was performed with Adobe Photoshop CS2 for panels A and B. Original magnification 10×/0.30 UPlanFL N lens for all panels.
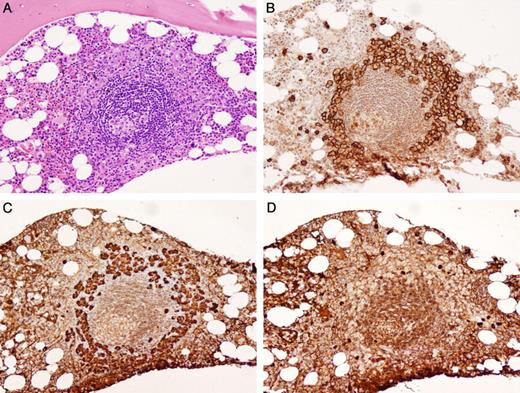
Lymphoid aggregates were identified in 33 (49%) pretreatment BM biopsies; 32 of the 33 cases had PC rimming of the aggregates (Figure 3). Of these, 24 were λ restricted, 1 was κ restricted, and 7 were polytypic PCs. Fewer PCs rimmed the lymphoid aggregates in the polytypic PC cases. The lymphoid aggregates themselves appeared histologically reactive and were composed of a mixed population of CD20-positive B cells and CD3-positive T cells. A series of control cases (20 normal, 35 LPL, and 47 PCPD) was examined to determine whether this histologic feature was unique to POEMS syndrome. Thirteen of 20 cases of normal BMs, and 7 of 47 cases of PCPD had lymphoid aggregates. However, the distinctive PC rimming of lymphoid aggregates was not found in any of the normal BMs or PCPD cases. In LPL, monoclonal PCs were found intermixed within B-cell aggregates. The PC rimming pattern was seen in 2 cases of LPL7 ; however, there was also a denser B-cell lymphocytic infiltrate as opposed to scattered small mixed B- and T-cell aggregates seen in POEMS cases.
Bone marrow biopsy of lymphoid aggregate rimmed by PCs. (A) The lymphoid aggregate has a regressed germinal center that is Castleman-like (H&E). (B) CD138 positive PCs form a distinctive rim around the lymphoid aggregate. (C) The PCs are monotypic for λ immunoglobulin light chains (D) and negative for κ immunoglobulin light chains by IHC. Photomicrographic images were obtained with an Olympus BX51 microscope equipped with an Olympus DP71 camera and software. Original magnification 20×/0.50 UPlanFL N lens for all panels.
Bone marrow biopsy of lymphoid aggregate rimmed by PCs. (A) The lymphoid aggregate has a regressed germinal center that is Castleman-like (H&E). (B) CD138 positive PCs form a distinctive rim around the lymphoid aggregate. (C) The PCs are monotypic for λ immunoglobulin light chains (D) and negative for κ immunoglobulin light chains by IHC. Photomicrographic images were obtained with an Olympus BX51 microscope equipped with an Olympus DP71 camera and software. Original magnification 20×/0.50 UPlanFL N lens for all panels.
Of 43 patients who presented with lymphadenopathy, 27 (63%) had lymphoid aggregates in the BM biopsy; 26 of the 27 were associated with PC rimming. Fifteen patients with lymphadenopathy had lymph node biopsies that showed morphologic features diagnostic of CD (n = 10) or had some CD-like features (n = 5). Eight of these patients also had lymphoid aggregates in their BM biopsies; 7 of the 8 had PCs surrounding the lymphoid aggregates. In one patient, there were histologic features in the BM biopsy similar to those seen in CD in the lymph node; there were lymphoid aggregates with regressed germinal centers and rimming by PCs (Figure 3). Given the association of CD with human herpes virus-8 (HHV8),10-12 IHC studies for HHV8 were performed on 32 cases (22 had lymphoid aggregates in the BM biopsy, and 13 had CD or CD-like features in lymph node biopsies). No case contained HHV8-positive cells.
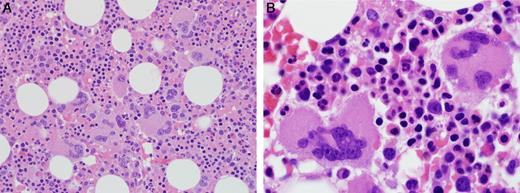
Twenty-four percent (16/67) of pretreatment BM specimens were hypercellular for age. Megakaryocytic hyperplasia was defined as an average of greater than 6 megakaryocytes per high power field and was present in 54% of cases. There was significant megakaryocytic clustering (5 or more clusters of 3 or more adjacent megakaryocytes without intervening cells) observed in 40% of cases. The megakaryocytes had atypical features, such as small mono- and hypolobated nuclei, hyperchromatic nuclei, separated nuclei, and/or large forms in 29 cases (Figure 4) and could have easily been interpreted as a myeloproliferative neoplasm (MPN) if the BMs had been evaluated in isolation. No significant reticulin fibrosis was present in any cases (n = 138). JAK2V617F testing were negative in all cases tested (n = 43), and no patients were found to have evolved to a MPN on follow-up.
Megakaryocyte clusters and atypical morphology. (Left) Megakaryocyte clusters were a common finding, original magnification ×400. (Right) Cytologically atypical megakaryocytes with abnormal nuclear segmentation and visible nucleoli. Photomicrographic images were obtained with an Olympus BX51 microscope equipped with an Olympus DP71 camera and software. Original magnifications: left panel, 40×/1.30 oil UPlanFL N lens; right panel, 100×/1.40 oil UPlanS Apo lens.
Megakaryocyte clusters and atypical morphology. (Left) Megakaryocyte clusters were a common finding, original magnification ×400. (Right) Cytologically atypical megakaryocytes with abnormal nuclear segmentation and visible nucleoli. Photomicrographic images were obtained with an Olympus BX51 microscope equipped with an Olympus DP71 camera and software. Original magnifications: left panel, 40×/1.30 oil UPlanFL N lens; right panel, 100×/1.40 oil UPlanS Apo lens.
The BM histopathologic findings in patients who had received therapy were similar to the BMs obtained from pretreatment patients (Table 2). Lymphoid aggregates with PC rimming were present but not as frequent, and the megakaryocytic hyperplasia was not as pronounced in the BM biopsies from the posttreatment patients. The finding of light-chain restricted PCs was also less common in this set of patients, undoubtedly because of the effects of chemotherapy. The persistence of these features may be beneficial in previously treated patients where a pretreatment BM is not available for review.
Discussion
Our data represent the largest BM morphologic study of patients with documented POEMS syndrome. The clinical recognition of POEMS syndrome is very challenging because there is wide variability in presenting symptoms and findings. As such, it is not uncommon for these patients to be initially labeled with a variety of neurologic, endocrine, or hematologic diagnoses, including MPN and/or MGUS. Even if a serum monoclonal protein is recognized in these patients, it can be easily passed off as a coexistent or “incidental” MGUS. Thus, in these situations it would not be uncommon for the BM histopathology, as described in this study, to provide the first indication that POEMS syndrome should be a diagnostic consideration.
Our study identified several unique and distinctive BM histopathologic features in patients with POEMS syndrome. When lymphoid aggregates were present, light chain-restricted PCs (usually λ light chain) frequently formed a distinct rim around the periphery of these aggregates. Fewer PCs surrounded the lymphoid aggregates in the occasional case in which the rimming PCs were polytypic. This distribution pattern of PCs relative to the lymphoid aggregate is unique and was not found in any of the control PCPD cases or normal BM cases and, although present in 2 of the 35 LPL cases, the associated dense B-cell lymphocytic infiltrate would morphologically raise the suspicion of lymphoma rather than a PC dyscrasia. Thus, when present, BM lymphoid aggregates rimmed by monotypic or polytypic PCs should lead the hematopathologist to raise the possibility of POEMS syndrome to the clinician.
A unifying feature of the POEMS cases was the presence of a monoclonal serum and/or urine protein in a polyclonal background,4 which was present in 95% of cases studied. This coexistence of clonal and polyclonal PCs was reflected in the BM which, in turn, made detection of the clonal PCs challenging. Although the methods and sophistication of the PC phenotyping modalities used during the course of the study varied, it seems likely that 6-color FC, through its capacity to simultaneously analyze surface antigen expression and cytoplasmic immunoglobulin light chain restriction, has a distinct advantage in this regard.
However, although we have previously demonstrated the superior sensitivity of 6-color FC compared with other methods used in this study, even this method did not prove 100% sensitive in detecting the clonal PCs of POEMS.8 This finding may reflect sampling bias possibly because of preferential aspiration of the normal (polytypic) PCs given the distinctive distribution of the clonal (monotypic) PC component in POEMS. Irrespective of the cause, given the apparent limitations of PC FC in this setting and the inherent ability of both IHC and ISH to both demonstrate immunoglobulin light chain restriction and illustrate the unique interrelationship of the monotypic PCs with the lymphoid aggregates, it appears that these methods are complimentary and that IHC or ISH should be considered in potential POEMS cases which lack detectable clonal PCs by FC.
As expected, because of the high incidence of thrombocytosis in this syndrome, megakaryocytic hyperplasia was present in more than one-half of the POEMS syndrome cases. What was less expected was that most of the BMs with the megakaryocytic hyperplasia also demonstrated megakaryocytic clustering and cytologic atypia, similar to that encountered in MPNs. The clinical presentation of peripheral thrombocytosis and/or erythrocytosis and splenomegaly resulted in an initial clinical consideration of a MPN in 3 patients before the BM examination, and the diagnosis of POEMS syndrome had been considered. Thus, it is critical that the full clinical and histopathologic picture be appreciated in these patients to avoid an incorrect diagnosis. From the pathology perspective, when a MPN is under consideration, the finding of either a clonal PC population or recognizing PC-rimmed lymphoid aggregates should lead the pathologist to consider possible POEMS syndrome. This is particularly true if the full spectrum of POEMS syndrome is not clinically appreciated at the time of the BM study. In the setting of erythrocytosis and/or thrombocytosis and a possible MPN, additional testing for serum erythropoietin and JAK2V617F mutation should be performed to adequately assess for that diagnostic consideration.
The finding of morphologic features reminiscent of CD in one of the BM specimens is also an unusual finding. BM findings in CD have been described in a case series by Kreft et al.13 In their report, 1 of 3 BM biopsies from patients with HIV-negative multicentric CD showed a lymphoid follicle with regressed germinal center surrounded by PCs. This patient also presented with hepatosplenomegaly, thrombocytosis, and goiter, 3 features that are commonly present in patients with POEMS syndrome. The finding of these features together with the unusual distribution of clonal PCs relative to the lymphoid aggregates suggests that the characteristic morphologic findings may be the result of immune dysfunction or dysregulation. These findings are unique to POEMS among the PC dyscrasias and it is highly unlikely that they are related to the paraprotein alone. The production of various cytokines such as IL-1β, IL-6, and TNF-α by the clonal PCs have been speculated to have a contributory role in this syndrome.14-18 VEGF is also likely to play a role in how this disease presents because many of the findings, such as organomegaly, edema, and skin changes, may be related to various angiogenic changes present in the affected tissues in patients with POEMS.17,19,20 It is known that platelets contain numerous proteins that regulate angiogenesis and is a major source of VEGF.21
In summary, this study has identified several histopathologic findings that, when present, appear to be unique to patients with POEMS syndrome. Although not all patients will exhibit all of the characteristic histologic features in their BM biopsies, the recognition of these features, in association with other clinical and laboratory data, should lead to the diagnostic consideration of POEMS syndrome. It is also very important in these patients that the finding of elevated peripheral blood counts, splenomegaly, and megakaryocyte clustering does not mistakenly lead to a diagnosis of a MPN. These distinctive morphologic findings raise interesting biologic questions regarding the etiology and clinical manifestations in this intriguing group of patients.
An Inside Blood analysis of this article appears at the front of this issue.
Presented in abstract form at the Annual Meeting of the United States and Canadian Academy of Pathology, Boston, MA, March 9, 2009.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health grant CA62242 and the Robert A. Kyle Hematologic Malignancies Fund.
National Institutes of Health
Authorship
Contribution: L.N.D. designed the research, performed experiments, analyzed data, and wrote the paper; C.A.H. designed the research, performed experiments, and edited the paper; A.D. designed the research, provided patient samples, made critical suggestions, and edited the paper; W.G.M. designed the research, performed experiments, and edited the paper; P.J.K. designed the research and edited the paper; and J.D.H. designed the research, supervised the study, performed experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James D. Hoyer, Department of Laboratory Medicine and Pathology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: jhoyer@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal