Li and colleagues are to be congratulated for conducting the first prospective clinical trial for POEMS syndrome, which treated 31 patients with 12 cycles of melphalan and dexamethasone.1 Although the choice of regimen is an old standard, this study is novel and important on several levels. First, it demonstrates that accrual for a trial for this very rare disease is possible. Second, the authors tackle response assessment in a multisystem disease. Third, it challenges what many consider to be the standard for treatment for this population of patients, albeit in the context of a prospective phase 2 trial.
This study reported in this issue of Blood raises more questions than it answers. The authors report an 81% hematologic response rate and improvement in neurologic function and serum VEGF in 100% of patients and suggest that these results, therefore, appear to be as good (and far less expensive than) high-dose chemotherapy with peripheral blood stem cell transplantation. A major caveat of this study, however, is short follow-up (21 months). In addition, this trial brings to the surface the importance of defining and standardizing response criteria for this disease. If one approaches hematologic response in POEMS syndrome as in multiple myeloma or immunoglobulin light chain (AL) amyloidosis, the first difficulty encountered is that the serum monoclonal protein studies do not perform as well in POEMS syndrome. The size of the M-protein is typically small, making standard multiple myeloma response criteria inapplicable in most cases. Moreover, although the immunoglobulin free light chains are elevated in 90% of POEMS patients, the ratio is normal in all but 18%,2 making the test that revolutionalized response assessment in AL amyloidosis not particularly helpful in patients with POEMS syndrome. The final major limitation of measuring response by M-protein alone in POEMS syndrome is that patients can derive very significant clinical benefit in the absence of hematologic response.1,3
There are also challenges using vascular endothelial growth factor (VEGF) as a response criterion as Li and colleagues did. VEGF assays are not standardized. There is even disagreement about which measurement—serum or plasma—is preferred.4 This disagreement is not trivial given that the normal values for the former are upwards of 10-fold that of the latter and susceptible to extremes in platelet count. Finally, the use of VEGF as a measurement becomes meaningless if anti-VEGF antibodies are part of the therapy because they bring VEGF levels down immediately and have a half-life of approximately 20 days.
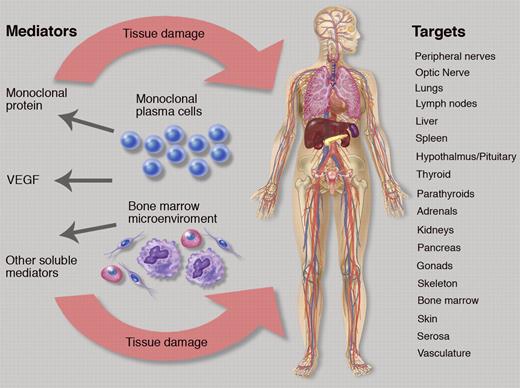
The figure alludes to the difficulty posed if one considers “organ response” or “clinical benefit,” when a multitude of organ systems are affected (see figure). To date, there are no defined criteria for responses or improvements of abnormalities for most of these parameters, and measurements are often relegated to the vague “improvement” or undefined “response” category. The most important symptom in POEMS syndrome is the peripheral neuropathy. Li et al used the Overall Neuropathy Limitations Scale (ONLS),5 which is a simple tool, but has been criticized as not providing adequate objective data—like nerve conduction studies—that are reproducible and are not subject to patient effort and/or volition.6 The ONLS does not distinguish between sensory, motor, or painful variants of neuropathy, but it provided information about the neuropathy status in the cohort of Li and colleagues. Most patients had a relatively mild peripheral neuropathy with a median ONLS of 4 (of 12), which could include one of the following constellations of symptoms: needing a walker to transverse 10 meters with no upper extremity symptoms; or needing a cane to transverse 10 meters and minor symptoms in the hands that caused no impairment in function. As this study demonstrates, the choice of a neuropathy score is a balance between detail of information gathered and feasibility of implementation.
The complex relationship of plasma cells, bone marrow microenvironment, soluble mediators, and target organ systems in POEMS syndrome. Professional illustration by Marie Dauenheimer.
The complex relationship of plasma cells, bone marrow microenvironment, soluble mediators, and target organ systems in POEMS syndrome. Professional illustration by Marie Dauenheimer.
If one steps through the figure and the potential systems that can be adversely affected by POEMS syndrome and therefore subject to improvement, there are more than 25 measurements that could be tracked when considering different facets of the neuropathy, organomegaly, endocrinopathy, skin changes, papilledema, extravascular volume overload, and pulmonary status. For most, there are no grading systems, but designations of “normal” and “abnormal” could be used for each. Alternatively, response criteria for POEMS syndrome could be abridged as follows: (1) hematologic response using a modified amyloid response criteria7 ; (2) VEGF response; (3) and a simplified organ response, which is limited to those systems causing the most morbidity, like peripheral neuropathy assessment, pulmonary function testing (diffusion capacity of carbon monoxide), and extravascular overload (grading ascites and pleural effusion as absent, mild, moderate, or severe).
Finally, this trial reminds us of how the study of the treatment of POEMS syndrome is in its infancy. In many ways, the medical profession's approach to the disease is not unlike what it was 35 years ago with AL amyloidosis, another complex, multisystem disease. The hematology community has come a long way with AL amyloidosis, most notably in the past decade, with the first consensus on definition of organ involvement and treatment response in AL amyloidosis published in 2005.7 High-dose chemotherapy with autologous stem cell transplantation (ASCT) was considered to be the best treatment for AL amyloidosis at the end of the 20th century, but that concept was challenged initially with a melphalan and dexamethasone trial in 20048 and later by a clinical trial that randomized patients between ASCT and melpahlan and dexamethasone in 2007.9 Alone, the work of Li and colleagues is not sufficient to rock the foundation for the role of ASCT in POEMS syndrome given the short follow-up of their patients, but it does put the spotlight on an orphan disease and serves as a platform for future clinical trials.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal