Abstract
The risk of Hodgkin lymphoma (HL) is increased in patients infected with HIV-1. We studied the incidence and outcomes of HL, and compared CD4+ T-cell trajectories in HL patients and controls matched for duration of combination antiretroviral therapy (cART). A total of 40 168 adult HIV-1–infected patients (median age, 36 years; 70% male; median CD4 cell count, 234 cells/μL) from 16 European cohorts were observed during 159 133 person-years; 78 patients developed HL. The incidence was 49.0 (95% confidence interval [CI], 39.3-61.2) per 100 000 person-years, and similar on cART and not on cART (P = .96). The risk of HL declined as the most recent (time-updated) CD4 count increased: the adjusted hazard ratio comparing more than 350 with less than 50 cells/μL was 0.27 (95% CI, 0.08-0.86). Sixty-one HL cases diagnosed on cART were matched to 1652 controls: during the year before diagnosis, cases lost 98 CD4 cells (95% CI, −159 to −36 cells), whereas controls gained 35 cells (95% CI, 24-46 cells; P < .0001). The incidence of HL is not reduced by cART, and patients whose CD4 cell counts decline despite suppression of HIV-1 replication on cART may harbor HL.
Introduction
HIV-1–infected patients have a risk of developing Hodgkin lymphoma (HL) that is approximately 10-fold higher than in the general population.1-14 Since the advent of potent combination antiretroviral therapy (cART), which suppresses the replication of HIV-1 and gradually restores immune function, the incidence of non-Hodgkin lymphoma and other AIDS-defining cancers has declined substantially.7,8,15 In contrast, the incidence of HL, a non–AIDS-defining cancer, does not appear to have decreased,16-19 with some studies finding that the incidence may have increased.8-11,20-22
There is debate on the relationship between immunodeficiency and the risk of HL in HIV-1–infected patients. The United States HIV/AIDS Cancer Match study20 found that patients with moderate immunodeficiency (CD4+ T-lymphocyte counts, 150-200 cells/μL) had the highest risk of developing HL, higher than patients with more severe immune suppression, supporting the hypothesis that some degree of immune competence may be required for HL to develop.23,24 In contrast, the French Hospital Database on HIV and others showed that the risk of HL increased as the CD4 cell declined.16,25,26 Lymphocytopenia at the time of diagnosis of HL is well documented in non–HIV-infected patients, and in these patients lymphocytopenia correlates with prognosis.27 It is, however, unclear when CD4+ cells and other lymphocytes start to decline in patients developing HL. This question can be examined in HIV cohort studies, which routinely monitor CD4 counts: a recent study in HIV-infected patients from Switzerland showed a decline of CD4 and CD8 cells in the year before the diagnosis.17,28
We examined the incidence and risk factors for HL and the CD4 cell count trajectories in HL patients and controls in the Collaboration of Observational HIV Epidemiological Research Europe (COHERE), a large collaborative effort of European HIV cohort studies.
Methods
COHERE
COHERE is a collaboration of 33 observational cohort studies of adult and pediatric HIV-infected patients in 30 European countries,29 which was established in 2005 with the objective of conducting hypothesis-driven clinical and epidemiologic research. All cohorts have been approved by local ethics committees or institutional review boards of all participating institutions, use standardized methods of data collection, and schedule follow-up visits at least once every 6 months. Cohorts transfer their data using the standardized HIV Collaboration Data Exchange Protocol29 to coordinating centers at the Copenhagen HIV Program, Copenhagen, Denmark, or the Institut de Santé Publique, d'Épidémiologie et de Développement, Bordeaux, France. Data collected include information on patient demographics, use of cART, CD4 cell counts, HIV-1 RNA concentration (viral load), AIDS-defining events and other complications, and deaths. Further information on COHERE is available at www.chip.dk/COHERE/tabid/295/Default.aspx and www.etudes.isped.u-bordeaux2.fr/cohere.
Inclusion criteria and definitions
We included data from cohorts that prospectively and systematically collected information on new diagnoses of HL in adults (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We included all cART-naive HIV-1–infected patients 16 years of age or older who started cART at some point after January 1, 1998, at a time when cART had become well established and widely used in Europe. All patients had to have at least one clinic visit after January 1, 1998 and before starting cART. In patients developing HL, the visit date had to be before the diagnosis of HL. Prevalent cases (ie, patients who were diagnosed with HL within one month after the first eligible visit date) were excluded.
Baseline CD4 cell count was defined as the first CD4 count measured during a visit after January 1, 1998. The baseline plasma HIV-1 RNA viral load was taken as the measurement closest to the baseline CD4 cell count, within a window of ± 30 days. The nadir CD4 cell count was defined as the lowest ever measured CD4 cell count up to 7 days after starting cART. In patients who developed HL before starting cART, the nadir CD4 count had to be measured before the diagnosis of HL. We defined cART as a regimen of at least 3 antiretroviral drugs from any drug class, including protease inhibitors, nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, and fusion inhibitors. The diagnosis of HL was based on excisional biopsy and histopathologic examination of tumor tissue. Data were merged on July 31, 2006.
Case-control studies of CD4 cell count trajectories in patients on cART
We performed 2 nested case-control studies in patients on cART to examine the evolution of peripheral CD4 cell counts before the diagnosis of HL. We included all patients who developed HL after starting cART. In the first study, all cases were included, with controls matched to cases for cohort, age at the start of cART (16-29, 30-39, 40-49, and ≥ 50 years), sex, transmission risk group (injection drug user, sex between men, heterosexual sex, other/unknown), Centers for Disease Control (CDC) clinical stage, and duration of cART. For each eligible HL case, we randomly selected up to 30 controls. In the second study, we restricted the analysis to patients who had been on cART for at least 90 days, with suppressed HIV-1 RNA viral load at the reference date (date of diagnosis of HL in cases or the date of identical length of follow-up since starting cART in controls), and who were free of AIDS. AIDS-free controls were matched to HL cases for CD4 cell count at the start of cART (< 50, 50-199, 200-349, and ≥ 350 cells/μL) and cohort, age, sex, transmission risk group, duration of cART treatment as defined in “Inclusion criteria and definitions.” For each eligible HL case, we randomly selected up to 5 controls. In both studies, we analyzed CD4 cell counts at 0, 180, 360, 540, and 720 days after start of cART, and at 0, 180, 360, and 540 days before the reference date. We allowed a window of ± 90 days for all time points, except for the reference date, where the time window was −90 to +7 days.
Statistical analysis
Incidence rates were calculated by dividing the number of patients developing the disease by the number of person-years at risk. In patients not on cART, we measured time from the date of the first visit after January 1, 1998 (baseline) until the date of diagnosis, start of cART, or the last follow-up visit, whichever came first. In patients on cART, we measured time from the start of cART until the date of diagnosis or the last follow-up visit. We used an intent-to-continue-treatment approach and thus ignored subsequent changes to treatment, including treatment interruptions and terminations. Survival in patients diagnosed with HL was analyzed by measuring time from the date of diagnosis to death from any cause or to the date the patient was last known to be alive.
We identified risk factors for HL in Weibull models, with random effects to account for the heterogeneity between cohort studies. Several cohorts had not recorded any HL cases. We therefore grouped cohorts into 4 regions (France, Western Europe other than France, Southern Europe, and other Europe) and included a random effect on region. CD4 cell counts and viral load were included as time-updated variables in some models. The slope of CD4 cell counts after the start of cART and during the year before the reference date was analyzed in multilevel linear regression models, and P values for differences between slopes in cases and controls were calculated. We determined the area under the viral load curve to quantify levels of exposure to HIV-1 replication over time. For each period between 2 viral load measurements, we calculated the area under the viral load curve by multiplying the log of the mean viral load by the number of days between measurements. Periods between 2 undetectable viral load measurements had an area under the viral load curve of zero. We calculated the total area under the viral load curve for each patient by adding up values of all periods.
Results are presented as medians with interquartile ranges (IQRs), incidence rates per 100 000 person-years, Kaplan-Meier estimates of the cumulative incidence of HL, crude and adjusted hazard ratios, as well as Kaplan-Meier probabilities of death with 95% confidence intervals (CIs). All analyses were done in Stata (Version 10, StataCorp, College Station, TX).
Results
Characteristics of cohorts and patients
The database included a total of 67 659 patients from 30 cohorts, including 107 patients who had been diagnosed with HL. Cohorts that did not actively ascertain HL were excluded from the analysis (13 cohorts with 21 503 patients). Another 5988 patients were excluded for the reasons detailed in Figure 1. Compared with included patients, excluded patients were more likely to be men (74% vs 70%), and men who have sex with men (34% vs 26%). Included and excluded patients did not differ in terms of CDC clinical stage, median age, and baseline CD4 and HIV-1 RNA values.
Identification of study population. The flow diagram shows the number of included and excluded patients.
Identification of study population. The flow diagram shows the number of included and excluded patients.
Data from 40 168 patients (59%) were analyzed. Median age was 36.1 years (IQR, 30.8-42.7 years), and most patients were male (28 085; 70%). Heterosexual contact was the most frequent risk factor for HIV-1 transmission, followed by sex between men and injection drug use. The median baseline CD4 cell count was 234 cells/μL (IQR, 96-400 cells/μL), the median nadir CD4 cell count was 198 cells/μL (IQR, 83-312 cells/μL), and the median plasma viral load was 74 300 copies/mL (IQR, 18 450-227 000 copies/mL). Table 1 compares the 78 patients who developed HL with the 40 090 patients who did not. HL patients were more likely to be male and had lower baseline CD4 cell counts and higher baseline viral loads.
Patient demographics and characteristics measured at baseline
| . | Patients developing HL (n = 78) . | Patients free of HL (n = 40 090) . |
|---|---|---|
| Median age, y (IQR) | 36.4 (33.1-41.3) | 36.1 (30.7-42.7) |
| Age, y, n (%) | ||
| 16-29 | 13 (17) | 8815 (22) |
| 30-39 | 41 (53) | 17 869 (45) |
| 40-49 | 12 (15) | 8668 (22) |
| At least 50 | 12 (15) | 4738 (12) |
| Sex, n (%) | ||
| Women | 19 (24) | 12 064 (30) |
| Men | 59 (76) | 28 026 (70) |
| Transmission risk group, n (%) | ||
| Injection-drug use | 17 (22) | 7642 (19) |
| MSM | 24 (31) | 10 592 (26) |
| Heterosexual | 22 (28) | 17 462 (44) |
| Other/unknown | 15 (19) | 4394 (11) |
| CDC clinical stage, n (%) | ||
| A/B | 64 (82) | 33 376 (83) |
| C | 14 (18) | 6714 (17) |
| Median CD4 cell count, cells/μL (IQR) | 170 (97-317) | 234 (96-400) |
| CD4 cell count, n (%) | ||
| < 50 cells/μL | 10 (13) | 5621 (14) |
| 50-99 cells/μL | 8 (10) | 3546 (9) |
| 100-199 cells/μL | 20 (26) | 6423 (16) |
| 200-349 cells/μL | 14 (18) | 8942 (22) |
| ≥ 350 cells/μL | 17 (22) | 11 247 (28) |
| Missing | 9 (12) | 4311 (11) |
| Median plasma HIV-1 RNA, copies/mL (IQR) | 100 340 (24 698-259 445) | 74 200 (18 437-227 000) |
| Plasma HIV-1 RNA, n (%) | ||
| ≥ 500 000 copies/mL | 9 (12) | 3956 (10) |
| 100 000-499 999 copies/mL | 22 (28) | 9371 (23) |
| 10 000-99 999 copies/mL | 21 (27) | 12 331 (31) |
| 501-9999 copies/mL | 8 (10) | 4992 (12) |
| ≤ 500 copies/mL | 0 (0) | 323 (1) |
| Missing | 18 (23) | 9117 (23) |
| . | Patients developing HL (n = 78) . | Patients free of HL (n = 40 090) . |
|---|---|---|
| Median age, y (IQR) | 36.4 (33.1-41.3) | 36.1 (30.7-42.7) |
| Age, y, n (%) | ||
| 16-29 | 13 (17) | 8815 (22) |
| 30-39 | 41 (53) | 17 869 (45) |
| 40-49 | 12 (15) | 8668 (22) |
| At least 50 | 12 (15) | 4738 (12) |
| Sex, n (%) | ||
| Women | 19 (24) | 12 064 (30) |
| Men | 59 (76) | 28 026 (70) |
| Transmission risk group, n (%) | ||
| Injection-drug use | 17 (22) | 7642 (19) |
| MSM | 24 (31) | 10 592 (26) |
| Heterosexual | 22 (28) | 17 462 (44) |
| Other/unknown | 15 (19) | 4394 (11) |
| CDC clinical stage, n (%) | ||
| A/B | 64 (82) | 33 376 (83) |
| C | 14 (18) | 6714 (17) |
| Median CD4 cell count, cells/μL (IQR) | 170 (97-317) | 234 (96-400) |
| CD4 cell count, n (%) | ||
| < 50 cells/μL | 10 (13) | 5621 (14) |
| 50-99 cells/μL | 8 (10) | 3546 (9) |
| 100-199 cells/μL | 20 (26) | 6423 (16) |
| 200-349 cells/μL | 14 (18) | 8942 (22) |
| ≥ 350 cells/μL | 17 (22) | 11 247 (28) |
| Missing | 9 (12) | 4311 (11) |
| Median plasma HIV-1 RNA, copies/mL (IQR) | 100 340 (24 698-259 445) | 74 200 (18 437-227 000) |
| Plasma HIV-1 RNA, n (%) | ||
| ≥ 500 000 copies/mL | 9 (12) | 3956 (10) |
| 100 000-499 999 copies/mL | 22 (28) | 9371 (23) |
| 10 000-99 999 copies/mL | 21 (27) | 12 331 (31) |
| 501-9999 copies/mL | 8 (10) | 4992 (12) |
| ≤ 500 copies/mL | 0 (0) | 323 (1) |
| Missing | 18 (23) | 9117 (23) |
MSM indicates men who have sex with men; and baseline, first visit after January 1, 1998.
Incidence rates and risk factors for developing HL
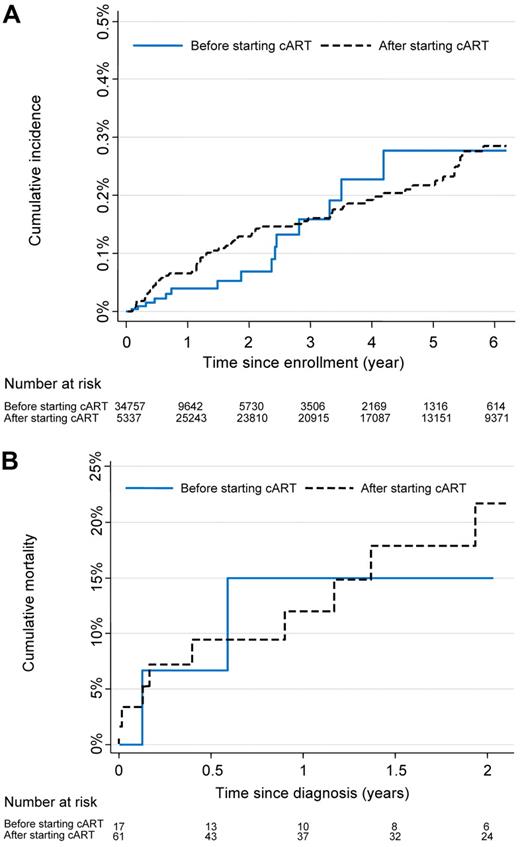
Table 2 shows the number of HL cases, person-years of follow-up, incidence rates, and crude and adjusted hazard ratios. Overall, the incidence rate was 49.0 (95% CI, 39.3-61.2) per 100 000 person-years. It was similar in patients not on cART and patients on cART: the crude hazard ratio (HR) was 1.02 (95% CI, 0.59-1.75). Median time from the start of observation to the diagnosis of HL was 2.37 years (IQR, 0.64-3.31 years) while not on cART and 1.50 years (IQR, 0.59-3.41 years) while on cART. The cumulative incidence of HL was 0.28% (95% CI, 0.21%-0.36%) by year 6 (Figure 2A).
Incidence of HL and baseline risk factors
| . | No. of cases . | Person-years at risk . | Incidence rate per 105 patient-years (95% CI) . | Crude HR (95% CI) . | P . | Adjusted HR (95% CI) . | P . |
|---|---|---|---|---|---|---|---|
| All patients | 78 | 159 133 | 49.0 (39.3-61.2) | — | — | — | — |
| On combination ART | .96 | ||||||
| No | 17 | 33 752 | 50.4 (31.3-81.0) | 1 | — | — | |
| Yes | 61 | 125 381 | 48.7 (37.9-62.5) | 1.02 (0.59-1.75) | — | — | |
| Age, y | .24 | .44 | |||||
| 16-29 | 13 | 35 348 | 36.8 (21.4-63.3) | 1 | 1 | ||
| 30-39 | 41 | 73 851 | 55.5 (40.9-75.4) | 1.53 (0.82-2.85) | 1.13 (0.59-2.16) | ||
| 40-49 | 12 | 32 380 | 37.1 (21.0-65.3) | 0.98 (0.45-2.15) | 0.68 (0.29-1.58) | ||
| ≥ 50 | 12 | 17 554 | 68.4 (38.8-120.4) | 1.82 (0.83-4.00) | 1.33 (0.58-3.05) | ||
| Sex | .27 | .71 | |||||
| Women | 19 | 47 238 | 40.2 (25.7-63.1) | 1 | 1 | ||
| Men | 59 | 111 894 | 52.7 (40.9-68.1) | 1.33 (0.79-2.25) | 1.13 (0.61-2.09) | ||
| Transmission group | .017 | .082 | |||||
| Injection-drug use | 17 | 31 160 | 54.6 (33.9-87.8) | 1.93 (1.01-3.68) | 1.58 (0.79-3.25) | ||
| MSM | 24 | 44 507 | 53.9 (36.1-80.5) | 1.66 (0.93-2.96) | 1.39 (0.71-2.74) | ||
| Heterosexual | 22 | 66 802 | 32.9 (21.7-50.0) | 1 | 1 | ||
| Other/unknown | 15 | 16 664 | 90.0 (54.3-149.3) | 2.83 (1.47-5.46) | 2.48 (1.24-4.99) | ||
| CDC clinical stage | .35 | .55 | |||||
| Stage A/B | 64 | 136 298 | 47.0 (36.8-60.0) | 1 | 1 | ||
| Stage C | 14 | 22 284 | 61.3 (36.3-103.5) | 1.33 (0.74-2.37) | 1.23 (0.63-2.39) | ||
| Baseline CD4 count, cells/μL | .042 | .051 | |||||
| < 50 | 10 | 19 595 | 51.0 (27.5-94.8) | 1 | 1 | ||
| 50-99 | 8 | 12 420 | 64.4 (32.2-128.8) | 1.26 (0.50-3.18) | 1.31 (0.51-3.35) | ||
| 100-199 | 20 | 23 145 | 86.4 (55.7-133.9) | 1.69 (0.79-3.60) | 1.81 (0.82-3.99) | ||
| 200-349 | 14 | 34 321 | 40.8 (24.2-68.9) | 0.80 (0.35-1.80) | 0.88 (0.37-2.09) | ||
| ≥ 350 | 17 | 53 472 | 31.8 (19.8-51.1) | 0.63 (0.29-1.37) | 0.69 (0.29-1.60) | ||
| Nadir CD4 count, cells/μL | .66 | ||||||
| < 50 | 10 | 22 443 | 44.6 (24.0-82.8) | 1 | — | — | |
| 50-99 | 10 | 14 703 | 68.0 (36.6-126.4) | 1.53 (0.64-3.67) | — | — | |
| 100-199 | 16 | 30 757 | 52.0 (31.9-84.9) | 1.16 (0.53-2.57) | — | — | |
| 200-349 | 18 | 44 418 | 40.5 (25.5-64.3) | 0.91 (0.42-1.96) | — | — | |
| ≥ 350 | 12 | 32 020 | 37.5 (21.3-66.0) | 0.84 (0.36-1.95) | — | — | |
| Baseline plasma HIV-1 RNA, copies/mL | .34 | ||||||
| ≥ 500 000 | 9 | 13 819 | 65.1 (33.9-125.2) | 1 | — | — | |
| 100 000-499 999 | 22 | 34 408 | 63.9 (42.1-97.1) | 0.99 (0.45-2.14) | — | — | |
| 10 000-99 999 | 21 | 49 856 | 42.1 (27.5-64.6) | 0.65 (0.30-1.41) | — | — | |
| 501-9999 | 8 | 22 968 | 34.8 (17.4-69.6) | 0.53 (0.21-1.38) | — | — | |
| ≤ 500 | 0 | 1078 | — | — | — | — |
| . | No. of cases . | Person-years at risk . | Incidence rate per 105 patient-years (95% CI) . | Crude HR (95% CI) . | P . | Adjusted HR (95% CI) . | P . |
|---|---|---|---|---|---|---|---|
| All patients | 78 | 159 133 | 49.0 (39.3-61.2) | — | — | — | — |
| On combination ART | .96 | ||||||
| No | 17 | 33 752 | 50.4 (31.3-81.0) | 1 | — | — | |
| Yes | 61 | 125 381 | 48.7 (37.9-62.5) | 1.02 (0.59-1.75) | — | — | |
| Age, y | .24 | .44 | |||||
| 16-29 | 13 | 35 348 | 36.8 (21.4-63.3) | 1 | 1 | ||
| 30-39 | 41 | 73 851 | 55.5 (40.9-75.4) | 1.53 (0.82-2.85) | 1.13 (0.59-2.16) | ||
| 40-49 | 12 | 32 380 | 37.1 (21.0-65.3) | 0.98 (0.45-2.15) | 0.68 (0.29-1.58) | ||
| ≥ 50 | 12 | 17 554 | 68.4 (38.8-120.4) | 1.82 (0.83-4.00) | 1.33 (0.58-3.05) | ||
| Sex | .27 | .71 | |||||
| Women | 19 | 47 238 | 40.2 (25.7-63.1) | 1 | 1 | ||
| Men | 59 | 111 894 | 52.7 (40.9-68.1) | 1.33 (0.79-2.25) | 1.13 (0.61-2.09) | ||
| Transmission group | .017 | .082 | |||||
| Injection-drug use | 17 | 31 160 | 54.6 (33.9-87.8) | 1.93 (1.01-3.68) | 1.58 (0.79-3.25) | ||
| MSM | 24 | 44 507 | 53.9 (36.1-80.5) | 1.66 (0.93-2.96) | 1.39 (0.71-2.74) | ||
| Heterosexual | 22 | 66 802 | 32.9 (21.7-50.0) | 1 | 1 | ||
| Other/unknown | 15 | 16 664 | 90.0 (54.3-149.3) | 2.83 (1.47-5.46) | 2.48 (1.24-4.99) | ||
| CDC clinical stage | .35 | .55 | |||||
| Stage A/B | 64 | 136 298 | 47.0 (36.8-60.0) | 1 | 1 | ||
| Stage C | 14 | 22 284 | 61.3 (36.3-103.5) | 1.33 (0.74-2.37) | 1.23 (0.63-2.39) | ||
| Baseline CD4 count, cells/μL | .042 | .051 | |||||
| < 50 | 10 | 19 595 | 51.0 (27.5-94.8) | 1 | 1 | ||
| 50-99 | 8 | 12 420 | 64.4 (32.2-128.8) | 1.26 (0.50-3.18) | 1.31 (0.51-3.35) | ||
| 100-199 | 20 | 23 145 | 86.4 (55.7-133.9) | 1.69 (0.79-3.60) | 1.81 (0.82-3.99) | ||
| 200-349 | 14 | 34 321 | 40.8 (24.2-68.9) | 0.80 (0.35-1.80) | 0.88 (0.37-2.09) | ||
| ≥ 350 | 17 | 53 472 | 31.8 (19.8-51.1) | 0.63 (0.29-1.37) | 0.69 (0.29-1.60) | ||
| Nadir CD4 count, cells/μL | .66 | ||||||
| < 50 | 10 | 22 443 | 44.6 (24.0-82.8) | 1 | — | — | |
| 50-99 | 10 | 14 703 | 68.0 (36.6-126.4) | 1.53 (0.64-3.67) | — | — | |
| 100-199 | 16 | 30 757 | 52.0 (31.9-84.9) | 1.16 (0.53-2.57) | — | — | |
| 200-349 | 18 | 44 418 | 40.5 (25.5-64.3) | 0.91 (0.42-1.96) | — | — | |
| ≥ 350 | 12 | 32 020 | 37.5 (21.3-66.0) | 0.84 (0.36-1.95) | — | — | |
| Baseline plasma HIV-1 RNA, copies/mL | .34 | ||||||
| ≥ 500 000 | 9 | 13 819 | 65.1 (33.9-125.2) | 1 | — | — | |
| 100 000-499 999 | 22 | 34 408 | 63.9 (42.1-97.1) | 0.99 (0.45-2.14) | — | — | |
| 10 000-99 999 | 21 | 49 856 | 42.1 (27.5-64.6) | 0.65 (0.30-1.41) | — | — | |
| 501-9999 | 8 | 22 968 | 34.8 (17.4-69.6) | 0.53 (0.21-1.38) | — | — | |
| ≤ 500 | 0 | 1078 | — | — | — | — |
Analyses are based on 40 168 HIV-infected patients from 16 European cohort studies. HRs are from random-effects Weibull regression models, crude and adjusted for age, sex, risk group, CDC clinical stage, and baseline CD4 cell counts. P values are from Wald tests.
ART indicates antiretroviral therapy; MSM, men who have sex with men; baseline, first visit after January 1, 1998; and —, not applicable.
Incidence and mortality of HL in HIV-infected patients. (A) Cumulative incidence of HL. (B) Cumulative mortality in patients with HL.
Incidence and mortality of HL in HIV-infected patients. (A) Cumulative incidence of HL. (B) Cumulative mortality in patients with HL.
In the multivariable analysis adjusted for age, sex, risk group, CDC clinical stage, and baseline CD4 cell count, there was some evidence that the risk of HL was higher in patients with CD4 cell counts between 100 and 199 cells/μL at the start of the observation compared with patients with CD4 cell counts less than 100 cells/μL or more than 200 cells/μL (P = .051, Table 2). The risk was also increased in patients with transmission group other or unknown, and in patients with a history of injection-drug use, but the association failed to reach statistical significance (P = .082). There was weak evidence of a higher risk in younger and older patients, compared with middle-aged patients (P = .44).
Risk factors were similar when restricting analyses to patients on cART, with one exception: patients with CDC clinical stage C at the start of cART were more likely to develop HL compared with patients in stages A or B. The crude HR was 1.98 (95% CI, 1.18-3.35) and the adjusted HR 1.46 (95% CI, 0.77-2.77). There was no evidence for a difference in risk across cART regimens (protease inhibitor-based, non-nucleoside reverse transcriptase inhibitor-based or nucleoside reverse transcriptase inhibitors only, data not shown). The number of patients who developed HL while not on cART was too small (n = 17) to allow separate analyses.
Models with time-updated CD4 cell count and HIV-1 RNA viral load
Table 3 shows hazard ratios for HL from multivariable models, including time-updated CD4 cell count and HIV-1 RNA viral load in patients before and after starting cART. There was a strong association with time-updated CD4 cell count, which remained when adjusted for time-updated viral load: the lower the time-updated CD4 cell count, the higher the risk of HL. The fully adjusted HR for a time-updated CD4 count of 350 cells/μL or higher was 0.27 (95% CI, 0.08-0.86) compared with a CD4 count of less than 50 cells/μL. Conversely, there was little evidence for an association of time-updated viral load. Results were similar when restricting the analysis to patients on cART (data not shown).
HRs for HL from multivariable models
| . | Adjusted HR (95% CI) . | ||
|---|---|---|---|
| Model 1 . | Model 2 . | Model 3 . | |
| Time-updated CD4 count, cells/μL | |||
| < 50 | 1 | — | 1 |
| 50-99 | 0.71 (0.19–2.67) | — | 0.89 (0.22–3.59) |
| 100-199 | 0.80 (0.29–2.23) | — | 0.99 (0.31–3.10) |
| 200-349 | 0.50 (0.18–1.35) | — | 0.57 (0.18–1.79) |
| At least 350 | 0.21 (0.08–0.59) | — | 0.27 (0.08–0.86) |
| Time-update HIV-1 RNA, copies/mL | |||
| ≥ 100 000 | — | 1 | 1 |
| 10 000-99 999 | — | 1.63 (0.64–4.16) | 2.06 (0.79–5.36) |
| 501-9999 | — | 1.13 (0.41–3.06) | 1.54 (0.55–4.32) |
| ≤ 500 | — | 0.67 (0.27–1.63) | 0.98 (0.38–2.50) |
| . | Adjusted HR (95% CI) . | ||
|---|---|---|---|
| Model 1 . | Model 2 . | Model 3 . | |
| Time-updated CD4 count, cells/μL | |||
| < 50 | 1 | — | 1 |
| 50-99 | 0.71 (0.19–2.67) | — | 0.89 (0.22–3.59) |
| 100-199 | 0.80 (0.29–2.23) | — | 0.99 (0.31–3.10) |
| 200-349 | 0.50 (0.18–1.35) | — | 0.57 (0.18–1.79) |
| At least 350 | 0.21 (0.08–0.59) | — | 0.27 (0.08–0.86) |
| Time-update HIV-1 RNA, copies/mL | |||
| ≥ 100 000 | — | 1 | 1 |
| 10 000-99 999 | — | 1.63 (0.64–4.16) | 2.06 (0.79–5.36) |
| 501-9999 | — | 1.13 (0.41–3.06) | 1.54 (0.55–4.32) |
| ≤ 500 | — | 0.67 (0.27–1.63) | 0.98 (0.38–2.50) |
All models include patients before and after starting cART and adjusted for age, sex, transmission group, and CDC clinical stage. Model 1 additionally includes time-updated CD4 count, model 2 additionally includes time-updated HIV-1 RNA, and model 3 includes both time-updated CD4 count and HIV-1 RNA in addition to the baseline variables.
— indicates not applicable.
Case-control studies of CD4 cell count and viral load trajectories on cART
In the first study, the 61 patients who developed HL on cART were matched to 1652 controls. Table 4 shows the characteristics of cases and controls. As expected, cases and controls were closely similar with regard to the matching criteria (ie, duration of cART, age, sex, and risk group). At diagnosis, CD4 cell counts were lower in cases than in controls (median count, 193 cells/μL vs 383 cells/μL, P < .0001), and the area under the log viral load curve was greater in cases than in controls. CD4 cell counts increased on cART both in cases and controls with similar slopes: cases gained 175 cells (95% CI, 111-240 cells) per year compared with 144 cells (95% CI, 131-157 cells) per year in controls (P for difference in slopes = .99). Conversely, during the year preceding the diagnosis, cases lost 98 cells (95% CI, 159-36 cells) per year, whereas controls gained 35 cells (95% CI, 24-46 cells; P for difference in slopes < .0001).
Patient characteristics and changes in CD4+ T-lymphocyte counts among HL cases and controls
| . | Study based on all cases . | Study based on selected cases* . | ||||
|---|---|---|---|---|---|---|
| Cases (n = 61) . | Controls (n = 1652) . | P† . | Cases (n = 18) . | Controls (n = 79) . | P† . | |
| Age, y (IQR) | 37.2 (33.1-42.1)‡ | 36.8 (32.8-43.5)‡ | — | 39.0 (34.4-49.5)‡ | 38.9 (34.6-46.5)‡ | — |
| Men, n (%) | 48 (79)‡ | 1 325 (80)‡ | — | 15 (83)‡ | 66 (84)‡ | — |
| Transmission group, n (%) | ||||||
| Injection-drug use | 14 (23)‡ | 339 (21)‡ | — | 5 (28)‡ | 19 (24)‡ | — |
| MSM | 19 (31)‡ | 550 (33)‡ | — | 5 (28)‡ | 22 (28)‡ | — |
| Heterosexual | 17 (28)‡ | 455 (28)‡ | — | 5 (28)‡ | 23 (29)‡ | — |
| Other/unknown | 11 (18)‡ | 308 (19)‡ | — | 3 (17)‡ | 15 (19)‡ | — |
| Duration of combination antiretroviral therapy, y (IQR) | 1.50 (0.59-3.41)‡ | 1.50 (0.56-3.36)‡ | — | 1.51 (1.03-3.36)‡ | 1.41 (1.03-3.36)‡ | — |
| AIDS at start of ART, n (%) | 18 (30)§ | 349 (21)§ | .12§ | 0‡ | 0‡ | — |
| Suppressed HIV-1 RNA load at reference date, n (%) | 35/48 (73)§ | 1 233/1 652 (75)§ | .79§ | 18/18 (100)‡ | 79/79 (100)‡ | — |
| CD4 count at start cART, cells/μL (IQR) | 167 (73-305)§ | 221 (87-372)§ | .15§ | 155 (86-259)‡ | 153 (82-272)‡ | — |
| Area under the log viral load curve (IQR) | 291 (58-1158)§ | 193 (0-718)§ | .045§ | 204 (43-316)§ | 192 (0-345)§ | .70§ |
| CD4 count at reference date, cells/μL (IQR) | 193 (55-328)§ | 383 (235-568)§ | .0001§ | 195 (76-268)§ | 369 (233-552)§ | .0004§ |
| CD4 count change per year after start cART, cells/μL (95% CI) | 175 (111-240)§ | 144 (131-157)§ | .99§ | 126 (62-189)§ | 140 (103-178)§ | .94§ |
| CD4 count change per year before reference date, cells/μL (95% CI) | −98 (−159 to −36)§ | 35 (24-46)§ | .0001§ | −99 (−196 to −1.5)§ | 59 (26-93)§ | .003§ |
| . | Study based on all cases . | Study based on selected cases* . | ||||
|---|---|---|---|---|---|---|
| Cases (n = 61) . | Controls (n = 1652) . | P† . | Cases (n = 18) . | Controls (n = 79) . | P† . | |
| Age, y (IQR) | 37.2 (33.1-42.1)‡ | 36.8 (32.8-43.5)‡ | — | 39.0 (34.4-49.5)‡ | 38.9 (34.6-46.5)‡ | — |
| Men, n (%) | 48 (79)‡ | 1 325 (80)‡ | — | 15 (83)‡ | 66 (84)‡ | — |
| Transmission group, n (%) | ||||||
| Injection-drug use | 14 (23)‡ | 339 (21)‡ | — | 5 (28)‡ | 19 (24)‡ | — |
| MSM | 19 (31)‡ | 550 (33)‡ | — | 5 (28)‡ | 22 (28)‡ | — |
| Heterosexual | 17 (28)‡ | 455 (28)‡ | — | 5 (28)‡ | 23 (29)‡ | — |
| Other/unknown | 11 (18)‡ | 308 (19)‡ | — | 3 (17)‡ | 15 (19)‡ | — |
| Duration of combination antiretroviral therapy, y (IQR) | 1.50 (0.59-3.41)‡ | 1.50 (0.56-3.36)‡ | — | 1.51 (1.03-3.36)‡ | 1.41 (1.03-3.36)‡ | — |
| AIDS at start of ART, n (%) | 18 (30)§ | 349 (21)§ | .12§ | 0‡ | 0‡ | — |
| Suppressed HIV-1 RNA load at reference date, n (%) | 35/48 (73)§ | 1 233/1 652 (75)§ | .79§ | 18/18 (100)‡ | 79/79 (100)‡ | — |
| CD4 count at start cART, cells/μL (IQR) | 167 (73-305)§ | 221 (87-372)§ | .15§ | 155 (86-259)‡ | 153 (82-272)‡ | — |
| Area under the log viral load curve (IQR) | 291 (58-1158)§ | 193 (0-718)§ | .045§ | 204 (43-316)§ | 192 (0-345)§ | .70§ |
| CD4 count at reference date, cells/μL (IQR) | 193 (55-328)§ | 383 (235-568)§ | .0001§ | 195 (76-268)§ | 369 (233-552)§ | .0004§ |
| CD4 count change per year after start cART, cells/μL (95% CI) | 175 (111-240)§ | 144 (131-157)§ | .99§ | 126 (62-189)§ | 140 (103-178)§ | .94§ |
| CD4 count change per year before reference date, cells/μL (95% CI) | −98 (−159 to −36)§ | 35 (24-46)§ | .0001§ | −99 (−196 to −1.5)§ | 59 (26-93)§ | .003§ |
Suppressed HIV-1 RNA load was defined as < 1000 copies/mL. The area under the log viral load curve was calculated for each period between 2 viral load measurements by multiplying the log of the mean viral load by the number of days between measurements and adding values across periods. Periods between 2 undetectable viral load measurements had an AUC of zero.
MSM indicates men having sex with men; and —, not applicable.
Patients free of AIDS who had been on cART for at least 90 days, with suppressed HIV-1 RNA viral load at the reference date (date of diagnosis of HL in cases or the date of identical length of follow-up since starting cART in controls).
P values for variables not used in matching cases and controls are shown (Kruskal-Wallis tests or χ2 tests).
Matched variables.
Unmatched variables.
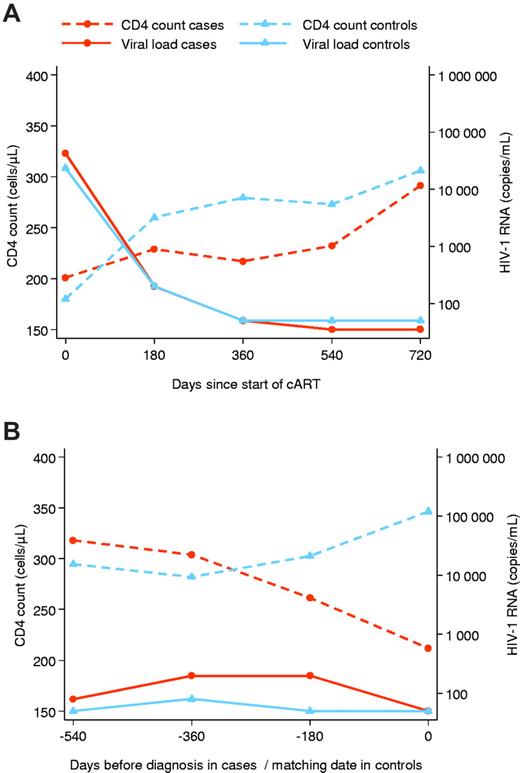
Eighteen cases (29.5%) met inclusion criteria for the second study and were matched to 79 controls. Forty-three patients (70.5%) were excluded because they had not been on cART for at least 90 days (n = 4), had an AIDS-defining condition (n = 28), or did not have the required CD4 cell count (n = 3) or viral load measurements (n = 8). Characteristics of patients included in the 2 case-control studies were similar (Table 4). CD4 cell counts at start of cART and at date of HL diagnosis CD4 cell counts at diagnosis were again lower in cases than in controls (median count, 195 cells/μL vs 369 cells/μL, P = .0004). Figure 3 shows trajectories of CD4 cell count and HIV-1 RNA viral load in cases and controls after starting cART (Figure 3A) and before the diagnosis of HL (Figure 3B). CD4 counts in cases decreased during the year preceding HL diagnosis (−99 cells per year; 95% CI, −196 to −1.5 cells), whereas controls continued to gain CD4 cells (59 cells per year; 95% CI, 26-93 cells; P for difference in slopes = .003). These differences were observed despite similar trajectories in viral load, both after starting cART (Figure 3A) and before the diagnosis of HL (Figure 3B), and overall similar areas under the log viral load curve (Table 4).
Evolution of CD4 cell counts and HIV-1 RNA viral load after the start of combination antiretroviral therapy and before the diagnosis of HL in cases and matched controls. Analysis is based on 18 HL cases and 79 matched controls. (A) Initial response to combination antiretroviral therapy. (B) Evolution before the date of diagnosis or corresponding reference date in controls.
Evolution of CD4 cell counts and HIV-1 RNA viral load after the start of combination antiretroviral therapy and before the diagnosis of HL in cases and matched controls. Analysis is based on 18 HL cases and 79 matched controls. (A) Initial response to combination antiretroviral therapy. (B) Evolution before the date of diagnosis or corresponding reference date in controls.
Patients developing HL before starting cART also experienced a decrease of CD4 cell counts in the year before HL diagnosis (−226 cells per year; 95% CI, −255 to −94 cells). However, in these untreated patients, the area under the log viral load curve was considerably greater (2291 copies/mL; IQR, 1990-2577 copies/mL) compared with patients receiving cART.
Survival
During a median follow-up of 18 months (IQR, 4.8-34.8 months), 12 of 78 patients diagnosed with HL died. Six (50%) died during the first 6 months of cART. Survival of patients was 88% (95% CI, 77%-94%) at one year and 81% (95% CI, 68%-89%) at 2 years (Figure 2B). There was little evidence for a difference in mortality for patients developing HL on cART or not on cART (P from log rank test = .73). Of note, all patients who were cART naive at HL diagnosis started cART after HL diagnosis; the median time between HL diagnosis and start cART in these patients was 10 days (IQR, 0-17 days). Given the small number of deaths, no further analyses were done to identify prognostic factors.
Discussion
This large collaborative analysis of European HIV-1 cohort studies found that the incidence of HL was close to 50 new cases per 100 000 person-years of follow-up, independently of whether or not patients received cART. Few risk factors could be identified. For example, HIV-1 viral replication at baseline was not associated with the risk of HL. There was some evidence that patients who started cART with advanced clinical disease were at increased risk of HL and that patients with baseline CD4 cell counts of 100 to 199 cells/μL were at higher risk of developing HL than patients with lower or higher CD4 cell counts, in line with the results from the United States HIV/AIDS Cancer Match study.20 In patients on cART, HL was associated with a loss of peripheral CD4 cells in the year before diagnosis, which was not explained by higher levels of HIV-1 replication. This was also reflected in time-updated analyses, which showed that the risk of HL increased as the most recent CD4 count decreased. Finally, 88% of HL patients were alive at one year after the diagnosis and 81% survived to 2 years.
Our study has several strengths and limitations. Thanks to a long-standing collaborative network of HIV cohort studies in Europe, data from more than 40 000 patients could be analyzed. CD4 cells and plasma HIV-1 RNA viral load were measured in regular intervals, which allowed us to examine the evolution of cell counts and levels of viral replication before the diagnosis of HL. The number of HL cases was reduced because not all cohorts systematically recorded this diagnosis during follow-up: HL is not classified as an AIDS-defining event. Even for cohorts systematically recording HL, data on Epstein-Barr Virus (EBV) serology, peripheral CD8 lymphocyte counts, histology, Ann Arbor stage, and chemotherapy regimen, which would have been of great interest in the present study, were not available. In addition, a central pathology review was not performed. Follow-up for patients diagnosed with HL was relatively short and less than 5 years on average. The relatively small number of cases meant that the power of our study was limited. For example, differences in the risk of HL across age groups, sex, or nadir CD4 count cannot be excluded with certainty. The case-control analysis of CD4 count trajectories in patients with suppressed HIV-1 viral loads was small because of stringent matching criteria. The close matching made it unlikely, however, that the differences we observed in the CD4 trajectories were explained by differences in HIV-1 replication or clinical stage. In addition, characteristics of patients included in this analysis were similar to the analysis based on all patients developing HL on cART. It is thus doubtful that a selection bias caused the observed CD4 decline.
The incidence of HL in HIV-1–infected patients observed in our study compares well with other studies from the cART era.15,17,20,25 A bimodal age distribution is characteristic for HL in HIV-negative patients,30 and some, albeit weak evidence of such bimodality was also found in this study and in previous analyses of single cohorts.17,25 A history of AIDS has been identified as a risk factor for HL in previous studies.9,31 In our study, this was evident only in patients on cART. Finally, it has been suggested that non-nucleoside reverse transcriptase inhibitors increase the risk to develop HL.21 However, this observation was not confirmed in other studies,32 or the present study. Survival of HIV-infected patients with HL was poor before cART became available but has improved considerably since then: survival is now approaching that of HIV-negative patients.33-38 A recent study of European HL patients diagnosed between 1995 and 1999 showed that, in HIV-negative men 15 to 44 years of age, survival at one year was 98%,39 whereas in our study the corresponding survival was 86% (95% CI, 73%-93%).
Our study and the HIV/AIDS Cancer Match study showed some evidence that patients with moderate immunosuppression at baseline were at the highest risk of HL.20 This is biologically plausible: in HL tissue, the majority of cells are benign lymphocytes, including CD4+ and CD8+ T lymphocytes, plasma cells, histiocytes, and eosinophil granulocytes, and only approximately 1% are malignant Reed-Sternberg cells.40,41 Reed-Sternberg cells express receptors, including CD40, that activate the nuclear factor-κB pathway.23,24,42 Ligands for these receptors are found on activated CD4 and other cells that surround Reed-Sternberg cells.23,24,42 Immune reconstitution after cART increases CD4 cell counts and possibly the interaction between CD40 and CD40L, which in turn may increase the risk of HL.24 EBV probably plays an important role in the pathogenesis of HL in HIV-1–infected patients: the majority of HL tissues in HIV-1–infected patients test positive for EBV and the EBV-encoded latent membrane protein 1 contributes to nuclear factorκB activation.23,24,42 If some degree of immune competence is required for HL to develop, then patients with moderate immunodeficiency should be at higher risk than patients with severe immunosuppression.24
Interestingly, when we examined the most recent CD4 T-lymphocyte count before HL diagnosis, the risk of HL increased substantially with decreasing CD4 cell counts. Similar observations were reported in the French Hospital Database on HIV,16,25 although this is not an independent observation as the French Hospital Database on HIV study participates in COHERE. In HIV-negative HL patients, lymphocytopenia is frequently observed at diagnosis and the degree of CD4 lymphocytopenia is associated with stage: with increasing Ann Arbor stage, the degree of peripheral lymphocytopenia increases, for CD20 B-lymphocytes, CD4 T–lymphocytes, CD8 T–lymphocytes, and NK cells (CD3−/CD56+/CD16+).42 Consequently, lymphocytopenia at HL diagnosis has been identified as a marker for poor survival.27 The mechanisms leading to peripheral lymphocytopenia are not well understood, and several factors, including immunosuppressive effects of cytokines and redistribution of lymphocytes from the periphery into tumor tissue, might be involved.41,43,44 Our results confirm previous observations from the Swiss HIV Cohort Study17,28 and indicate that declining CD4 cell counts in patients responding to cART may represent early signs of HL.45 Further research is required to clarify to what extent this observation is specific to HL, or a more general phenomenon in patients developing opportunistic events.46 Moreover, peripheral CD4 cell counts provide information on one aspect of immune function only: HIV infection probably affects the immune system in other, more complex ways that may be relevant to the pathogenesis of HL.47
In conclusion, this large collaborative analysis of European HIV cohort studies showed that, in the era of cART, the incidence of HL is similar in patients receiving cART and patients not on cART. In HIV-infected patients, a decline of CD4 cell counts, which is not explained by virologic failure of cART, may herald the diagnosis of HL and should therefore alert clinicians to the possibility of a developing HL.
The online version of this article contains a data supplement.
Presented as oral presentation at the 52nd Annual Meeting of the American Society of Hematology, Orlando, FL, December 8, 2009; oral presentation at the 12th International Conference on Malignancies in AIDS & Other Opportunistic Infections, Bethesda, MD, April 27, 2010; poster presentation at the 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, CA, February 18, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was performed within the framework of the COHERE study group. The lymphoma project was supported by a Swiss Bridge Foundation grant (M.E.). The COHERE study group has received generic funding from Agence Nationale de Recherches sur le SIDA et les Hépatites Virales, France; HIV Monitoring Foundation, The Netherlands; and the Augustinus Foundation, Denmark. The group has also received project specific funding from the United Kingdom Medical Research Council. A list of the funders of the participating cohorts can be found on the Regional Coordinating Center Web sites at www.cphiv.dk/COHERE/tabid/295/Default.aspx and www.etudes.isped.u-bordeaux2.fr/cohere. The study sponsors had no role in the design of the study, the collection, analysis and interpretation of data, the writing of the report, or in the decision to submit the paper for publication.
Authorship
Contribution: J.B. designed the study, performed statistical analyses, interpreted the findings, and drafted and submitted the manuscript; K.S. performed all statistical analyses and contributed to the interpretation of findings and review of the final manuscript for submission; F. Boué, G.F., M.M., A.M.C.-M., A.M., F. Bonnet, G. Clifford, V.P., J.M.M., N.O., M.P., and G. Chêne designed the study, chose the statistical analyses, interpreted the findings, and prepared and reviewed the final manuscript for submission; and M.E. designed the study, performed statistical analysis, drafted and revised the manuscript, and served as guarantor for the analyses with full access to the dataset.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of members of the COHERE study group appears in the supplemental Appendix.
Correspondence: Julia Bohlius, Institute for Social and Preventive Medicine, University of Bern, Finkenhubelweg 11, CH-3012 Bern, Switzerland; e-mail: jbohlius@ispm.unibe.ch.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal