Abstract
Reactive oxygen species (ROS) are a heterogeneous group of molecules that are generated by mature myeloid cells during innate immune responses, and are also implicated in normal intracellular signaling. Excessive production of ROS (and/or a deficiency in antioxidant pathways) can lead to oxidative stress, a state that has been observed in several hematopoietic malignancies including acute and chronic myeloid leukemias (AML and CML). Currently it is unclear what the cause of oxidative stress might be and whether oxidative stress contributes to the development, progression, or maintenance of these diseases. This article reviews the current evidence suggesting a role for ROS both in normal hematopoiesis and in myeloid leukemogenesis, and discusses the usefulness of therapeutically targeting oxidative stress in myeloid malignancy.
Introduction
Over the past 15 years, there has been a growing appreciation that reactive oxygen species (ROS) production plays an important role in a variety of cellular processes, in addition to their antimicrobial role during phagocytosis by cells of the innate immune system. Specifically, ROS generated by the mitochondria or nicotinamide adenine dinucleotide phosphate (NADPH) oxidases have been shown to influence cell-cycle progression, cell motility, and growth factor signaling in a variety of normal cell types.1
Many pathologic states are accompanied by excessive cellular ROS production and/or a deficiency in antioxidant defenses, leading to a state known as oxidative stress.2 Evidence for chronic oxidative stress has been found in many cancers, both in solid tumors such as prostate carcinoma3 and melanoma4 and in several hematopoietic malignancies including acute lymphoblastic leukemia (ALL),5 myelodysplastic syndrome (MDS),6 and myeloid leukemias including chronic myeloid leukemia (CML) and acute myeloid leukemia (AML).7 The importance of the association between oxidative stress and malignancy is not currently clear; however, there is evidence that tumor-derived ROS may promote cell survival,8-10 migration and metastasis,11,12 proliferation,13,14 and even drug-resistance,15 depending on the origin of the cancer. These observations suggest that oxidative stress may subvert the normal roles of ROS so as to benefit the malignant clone.
In the context of AML, a recent report indicates that relapse in this disease is associated with increased markers of oxidative stress within the leukemic blasts, suggesting that ROS production may be an important factor in AML progression.16 Indeed, recent published data from our group suggest that constitutively active Ras (one of the most common abnormalities detected in AML) is capable of driving ROS production in CD34+ human hematopoietic progenitor cells, and this significantly contributes to the mutant Ras phenotype.17 Furthermore, ROS production appears to contribute to proliferation and migration of hematopoietic cells expressing a variety of oncogenic tyrosine kinases.18 Thus, increased oxidative stress in leukemic cells may represent a potential therapeutic target, although there are differing opinions on whether therapeutic strategies should aim to antagonize or further promote oxidative stress in leukemic cells. This article will discuss recent advances in understanding the role of ROS in normal hematopoiesis and in myeloid leukemias and will also discuss whether ROS production presents a viable therapeutic target in myeloid malignancies.
Introduction to reactive oxygen species
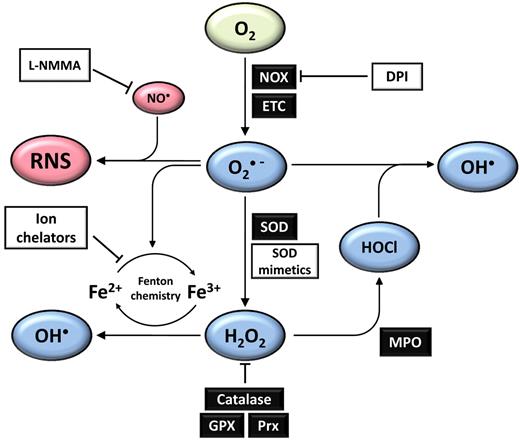
ROS are a heterogeneous group of molecules and free radicals derived from diatomic oxygen, with a wide spectrum of reactivity. In physiologic systems, ROS formation begins with the univalent reduction of oxygen to produce superoxide radicals (Figure 1), which lie at the hub of a variety of potential chemical reactions.19 For example, superoxide can dismutate to form hydrogen peroxide (H2O2), a membrane-permeable, mildly pro-oxidant molecule with putative roles as a second messenger. Further processing of H2O2 either via Fenton chemistry or by enzymatic catalysis can lead to formation of several highly oxidizing derivatives including hydroxyl radicals and hypochlorous acid (HOCl).20 Colocalization of ROS production (specifically superoxide) at sites of nitric oxide (NO∙) production can lead to formation of a distinct family of pro-oxidant molecules collectively known as reactive nitrogen species (RNS), the proximal species being peroxynitrite.19 Although RNS are not the focus of this review, it is important to be aware that formation of RNS may well contribute to the effects of ROS in physiologic systems. Overall, it is clear that production of ROS must be controlled in vivo, to avoid oxidative stress and accompanying oxidative damage.
Physiologic ROS homeostasis networks. Univalent reduction of oxygen results in the formation of superoxide (O2∙ −), which can occur as a result of NADPH oxidase (NOX) activity, and also as a by-product of oxidative phosphorylation, primarily at complex I in the mitochondrial electron transport chain (ETC). Superoxide may act as a reductant or an oxidant and is a key molecule in several subsequent physiologic reactions. Most of the superoxide generated in vivo is converted into hydrogen peroxide (H2O2) primarily by the actions of superoxide dismutases, which exist in cytosolic (SOD1), mitochondrial (SOD2), and extracellular (SOD3) isoforms. H2O2 levels are tightly regulated by several mechanisms, including the actions of catalase, the glutathione peroxidase (GPX) system, and peroxiredoxins (Prx). H2O2 may be further processed by the actions of myeloperoxidase (MPO) during an immune response to form hypochlorous acid (HOCl), which may in turn react with superoxide to form hydroxyl radicals. Hydroxyl radicals may also be formed from H2O2 by Fenton chemistry, which may occur in the presence of free metal cations such as Fe2+ or Cu+. Where superoxide production and production of nitric oxide (NO∙) are colocalized, reactive nitrogen species (RNS) may be formed, with the proximal species being peroxynitrite. Various RNS may then form via further chemical reactions with other ROS or RNS. This network of ROS and RNS production can be disrupted or biased in the presence of various compounds such as diphenyleneiodonium (DPI), which inhibits flavoproteins including the NOX oxidase family, ion chelators that can terminate Fenton chemistry cycles, and L-arginine analogs such as L-monomethyl arginine (L-NMMA), which inhibits nitric oxide synthase. Green represents molecular oxygen, blue are ROS derived from O2, and red represents nitric oxide and other RNS.
Physiologic ROS homeostasis networks. Univalent reduction of oxygen results in the formation of superoxide (O2∙ −), which can occur as a result of NADPH oxidase (NOX) activity, and also as a by-product of oxidative phosphorylation, primarily at complex I in the mitochondrial electron transport chain (ETC). Superoxide may act as a reductant or an oxidant and is a key molecule in several subsequent physiologic reactions. Most of the superoxide generated in vivo is converted into hydrogen peroxide (H2O2) primarily by the actions of superoxide dismutases, which exist in cytosolic (SOD1), mitochondrial (SOD2), and extracellular (SOD3) isoforms. H2O2 levels are tightly regulated by several mechanisms, including the actions of catalase, the glutathione peroxidase (GPX) system, and peroxiredoxins (Prx). H2O2 may be further processed by the actions of myeloperoxidase (MPO) during an immune response to form hypochlorous acid (HOCl), which may in turn react with superoxide to form hydroxyl radicals. Hydroxyl radicals may also be formed from H2O2 by Fenton chemistry, which may occur in the presence of free metal cations such as Fe2+ or Cu+. Where superoxide production and production of nitric oxide (NO∙) are colocalized, reactive nitrogen species (RNS) may be formed, with the proximal species being peroxynitrite. Various RNS may then form via further chemical reactions with other ROS or RNS. This network of ROS and RNS production can be disrupted or biased in the presence of various compounds such as diphenyleneiodonium (DPI), which inhibits flavoproteins including the NOX oxidase family, ion chelators that can terminate Fenton chemistry cycles, and L-arginine analogs such as L-monomethyl arginine (L-NMMA), which inhibits nitric oxide synthase. Green represents molecular oxygen, blue are ROS derived from O2, and red represents nitric oxide and other RNS.
Sources of ROS production in hematopoietic cells
Mitochondrial ROS
The mitochondrial electron transport chain (ETC) is a multicomponent system of redox-coupled proteins that are expressed in the inner mitochondrial membrane. The mitochondrial ETC is the means by which high-energy electrons donated by nicotinamide adenine dinucleotide (NADH) are harnessed to drive formation of adenosine 5′-triphosphate (ATP). The mitochondrial ETC is the subject of some excellent reviews,21,22 therefore, a detailed description of the components of the ETC is not included here; however, it is useful to note that the proximal enzyme in the ETC is the NADH dehydrogenase complex (also known as complex I). This complex accepts high-energy electrons donated by NADH, and appears to be the most common site of ROS production in the mitochondria. Studies using isolated mitochondria from mammalian cells suggest that electrons may escape from the ETC predominantly at complex I and univalently reduce O2, forming superoxide.23 Under these experimental conditions, it has been estimated that as much as 1%-2% of the oxygen consumed by isolated mammalian mitochondria is converted to superoxide.24 Mitochondrial ROS production depends on several factors such as the magnitude of the proton motive force, the local concentration of O2, whether the ETC has been damaged, and the ratios of both NADH/NAD+ and oxidized/reduced coenzyme Q.23 Because all of these factors are highly dynamic and variable in vivo, it is difficult to accurately predict the extent of ROS production by mitochondria in vivo, although it is likely to be considerably less than that observed in vitro.23 Finally, although measurement of mitochondrial ROS remains difficult in vivo, semi-quantitative methods of detecting mitochondrial ROS within intact cells have been developed.25,26
NOX family
NADPH oxidase (NOX) enzymes are a family of proteins that catalyze the univalent reduction of O2 to superoxide using NADPH as an electron donor and have been the subject of a recent extensive review.27 The first discovered member of this family (now known as NOX2) plays an important role in the destruction of pathogens by mature phagocytes, where it forms the catalytic subunit of the phagocyte NADPH oxidase complex (also known as Phox). The observation that some nonphagocytic cells could produce significant amounts of superoxide (even when deficient for NOX228 ) prompted the search for additional members of the NOX family. Currently, the NOX family of proteins consists of 7 members, NOX1-5 and dual oxidase (DUOX) 1 and 2, each with differing regulatory mechanisms. The first 4 members of the family (NOX1-4) all require association with p22Phox to ensure correct posttranslational modification, membrane targeting, and long-term stability. Furthermore, these proteins (with the exception of NOX4) require several additional regulatory subunits for activity, which must translocate from the cytosol to form a functional complex with membrane-bound NOX. NOX5 differs from other NOX family members in that it does not require association with p22Phox or regulatory subunits for activity, but instead contains an EF-hand domain, and appears to be regulated in part by Ca2+ ions.29 Finally, DUOX proteins also appear to be partly regulated by Ca2+ in a similar manner to NOX5, and also function without association with p22Phox. Instead, DUOX proteins require co-expression with dual oxidase maturation factors (DUOXA proteins), which appear to serve a similar role to p22Phox in that they facilitate correct posttranslational modification and targeting of DUOX to the plasma membrane.30 The known regulatory subunits for each NOX family member are summarized in Table 1.
Summary of NOX family proteins, human tissue distribution, and known regulatory factors
| Enzyme . | Tissue distribution* . | Known regulatory factors . |
|---|---|---|
| NOX1 | Colon epithelial cells, vascular smooth muscle | Primarily NOXO1, NOXA1, and p22Phox |
| NOX2 (gp91Phox) | Phagocytes | p22Phox, p47Phox, p67Phox, p40Phox, and Rac |
| NOX3 | Inner ear | p22Phox, other subunits may contribute |
| NOX4 | Kidney, vascular endothelial cells, vascular smooth muscle | p22Phox |
| NOX5 | Spleen, testis, vascular smooth muscle | Calcium |
| DUOX1 | Thyroid, lung, prostate, testis, salivary glands, pancreas | Calcium, DUOXA1 |
| DUOX2 | Thyroid, intestinal tract | Calcium, DUOXA2 |
| Enzyme . | Tissue distribution* . | Known regulatory factors . |
|---|---|---|
| NOX1 | Colon epithelial cells, vascular smooth muscle | Primarily NOXO1, NOXA1, and p22Phox |
| NOX2 (gp91Phox) | Phagocytes | p22Phox, p47Phox, p67Phox, p40Phox, and Rac |
| NOX3 | Inner ear | p22Phox, other subunits may contribute |
| NOX4 | Kidney, vascular endothelial cells, vascular smooth muscle | p22Phox |
| NOX5 | Spleen, testis, vascular smooth muscle | Calcium |
| DUOX1 | Thyroid, lung, prostate, testis, salivary glands, pancreas | Calcium, DUOXA1 |
| DUOX2 | Thyroid, intestinal tract | Calcium, DUOXA2 |
NOX1, 2, and 4 are also expressed in human CD34+ hematopoietic progenitor cells.
The tissue distribution of NOX family proteins varies greatly; for example, NOX1 is most highly expressed in the colon, NOX2 in mature phagocytes, NOX3 in the inner ear, NOX4 in the kidney, NOX5 in the spleen and testis, and DUOX proteins in the thyroid.27,29 However, each of these proteins (perhaps with the exception of NOX3) are also expressed at moderate to low levels in a variety of additional tissues (see Table 1). Furthermore, the effects of ROS production by NOX proteins are likely to be greatly affected not only by their tissue distribution but also by their subcellular location. However, the subcellular location of NOX proteins is not clearly defined in many cases. Indeed, evidence suggests that some family members (eg, NOX4, NOX1) can be found in the plasma membrane, in the ER, or even in the nucleus, depending on the context,27 although recently developed monoclonal antibodies are shedding more light on NOX4 localization.31 NOX2 is mostly located in the plasma membrane and phagosomes of maturing phagocytes, while DUOX proteins are reliably expressed in the apical plasma membrane of thyroid epithelial cells.29 The roles of several NOX family members have been established, including NOX2-based NADPH oxidases in immune defense,32 the importance of DUOX in thyroid function,33 and NOX3 for inner ear development.34 The requirement of specific NOX proteins in some contexts suggests that subtle quantities of ROS may be deliberately produced by cells in a variety of normal tissues. Together with growing evidence that some ROS act as second messengers, it has been suggested that NOX proteins play important roles in intracellular signaling. Interestingly, several of these NOX family members and their corresponding regulatory proteins are expressed and functional in the plasma membrane of human CD34+ hematopoietic progenitor cells,35 although it currently remains unclear what role NOX proteins play in this context.
Metabolic pathways
Several metabolic pathways are associated with ROS production including polyamine metabolism,36 cytochrome P450 activity,37 and xanthine oxidase activity.38 In the context of hematopoietic cell metabolism, a recent report has demonstrated that arachidonic acid (AA) metabolism plays an important role in murine hematopoietic stem cell (HSC) function and is capable of generating significant intracellular ROS.39 Mice deficient for 12/15 lipoxygenase (LOX, a key enzyme in the AA metabolism network40 ) showed defective HSC pool maintenance and function, which was accompanied by a decrease in 12/15 LOX products and changes in intracellular ROS levels, although the significance of ROS in this phenotype was not clarified.
ROS homeostasis and ROS signaling
Superoxide is the proximal ROS generated in hematopoietic cells. Uncontrolled superoxide production can lead to accumulation of more dangerous ROS, most notably the hydroxyl radical19 (Figure 1). As a consequence of their high reactivity, hydroxyl radicals (and other molecules such as HOCl and peroxynitrite) have very short half-lives and participate in oxidation of a broad range of cellular components. Hydroxyl radicals are particularly destructive and can abstract protons from the DNA backbone causing double-strand breaks (DSBs) or can react with guanine residues forming 8-oxo-guanine (an important mechanism in GC to AT transversion in genomic DNA).41 Hydroxyl radicals can also initiate lipid peroxidation chain reactions leading to disruption of lipid bilayers and lipid signaling molecules.1 Several isomers of prostaglandins (isoprostanes) are formed through the interaction of a free radical with arachidonic acid42 and act as reliable markers for ROS damage to lipids. Furthermore, lipid peroxidation products include carbonyl-containing lipid derivatives such as malondialdehyde (MDA), which can then go on to form adducts with proteins (either at the N-terminal amino group, or within threonine, proline, lysine, histidine, or arginine residues), forming carbonylated proteins.43 Protein carbonyls and MDA have been used to measure oxidative damage of proteins in hematologic malignancies.44,45 The potential toxicity of ROS (especially hydroxyl radicals) demands that ROS production is controlled and promptly terminated when not required. Accordingly, several ROS catabolizing enzymes and molecules are expressed by virtually all human cells for the purpose of eliminating ROS, and operate at key points within ROS reaction networks to control ROS production. Deficiencies in these mechanisms can lead to oxidative stress.
Superoxide dismutase (SOD) is the main enzyme responsible for eliminating superoxide before it can participate in the formation of other ROS and is therefore a key line of defense against oxidative stress. SOD catalyzes a dismutation reaction between 2 superoxide anions, forming one molecule of oxygen and one peroxide dianion, which is rapidly protonated to form H2O2.46 SOD isoforms are expressed in the cytosol (SOD1), mitochondria (SOD2), and even in the extracellular space (SOD3)20 ; they are thus positioned to detoxify superoxide soon after it is generated, which is particularly important in mitochondria. However, because of the actions of cellular SODs, H2O2 can rapidly accumulate at sources of superoxide production. Although H2O2 is a relatively stable member of the ROS family, it too can participate in oxidation reactions with proteins and lipids, and can also be a source of more reactive and toxic species especially in the presence of peroxidases or metal ions. There are several known mechanisms whereby H2O2 levels are regulated in eukaryotic cells, perhaps suggesting the importance of H2O2 homeostasis. The 3 best understood mechanisms of H2O2 regulation are via the actions of catalase, the glutathione system, and the peroxiredoxin (Prx) system. Catalase has one of the fastest turnover rates of any enzyme known (essentially limited only by the rate of diffusion),47 and efficiently catalyzes the conversion of 2 molecules of H2O2 to diatomic oxygen and 2 water molecules. However, catalase expression is usually restricted to peroxisomes in mammalian cells; therefore, H2O2 destruction in the cytosol is mainly achieved through the actions of glutathione peroxidase as part of the glutathione (GSH) system, and the Prx system. GSH is a tripeptide containing a central cysteine residue, and is the most abundant thiol in mammalian cells, with reported concentrations approaching 10mM.48 Glutathione peroxidase catalyzes the reduction of H2O2 to form oxygen and water, using GSH as an electron donor. Oxidation of GSH in this way results in the formation of oxidized GSH dimers (GSSG). GSH may also form mixed disulphides at cysteine residues within proteins, which may serve as a protective mechanism against further cysteine oxidation.49 GSH is regenerated from GSSG dimers or mixed disulphides by glutathione reductase in a process that uses electrons donated by NADPH or NADH.50 Finally, the Prx family of proteins provides a further detoxification mechanism defense against H2O2, reviewed by Rhee et al.51 Prxs are homodimeric cytosolic proteins containing a conserved N-terminal cysteine residue (Cys51 in mammalian Prx), which is sacrificially oxidized by H2O2, eliminating H2O2 in the process. In 2-cys Prx family members, the oxidized cysteine residue on the first subunit then forms an intermolecular disulphide bond with the second subunit. This disulphide bond is resolved by the activity of thioredoxin (TRX), hence 2-cys Prxs can participate in further oxidation reactions and catalytically destroy H2O2.51 The proteins that govern the cycles of Prx oxidation and regeneration are also able to regenerate oxidized cysteine residues in several other protein targets, and these cysteine oxidation cycles form the foundation of our current understanding of ROS signaling. Clearly, it is of utmost importance that excessive ROS production is controlled to avoid widespread toxicity. However, total elimination of ROS has also been shown to be deleterious to a variety of cell types, suggesting that ROS may act as signaling molecules in some contexts. The first evidence that ROS may play signaling roles was obtained during the late 1980s and early 1990s, when it was observed that exogenous superoxide and H2O2 appeared to promote cell proliferation in a variety of contexts including human U937 monoblastic leukemia cells,52 hamster BHK-21 cells, and rat 208F fibroblast cell lines,53-55 and shortly afterward in human fibroblasts and amnion cells.56,57 Subsequent studies demonstrated that platelet-derived growth factor (PDGF),58 epidermal growth factor (EGF),59 and angiotensin II signals60 were dependent on downstream H2O2 production, suggesting that ROS production may be an important feature of normal growth factor receptor signaling. In addition, several hematopoietic growth factor signals were shown to promote ROS production and cell-cycle progression, and blockade of growth factor–induced ROS suppressed proliferation and viability.61,62
H2O2 signaling has been implicated in diverse processes including cell-transformation, senescence, apoptosis, and extracellular matrix remodeling; this has been discussed in some excellent reviews.2,20,63,64 H2O2 is uncharged and membrane permeable and has a relatively low reactivity (compared with hydroxyl radicals or HOCl), and a conserved antioxidant network exists (including the GSH and Prx systems) that can efficiently eliminate it. All of these characteristics make H2O2 capable of acting as a second messenger. The best understood mechanism of H2O2 signaling is via the reversible oxidation of cysteine residues, which appears to play in role in regulating the activity of several phosphatases.65 For example, protein tyrosine phosphatases (PTPs) such as PTP1B are well-established targets for inactivation by H2O2 via oxidation of a critical cysteine residue in the active site.66 However, recent evidence suggests that the lipid phosphatase phosphatase and tensin homolog (PTEN)51 and even some serine/threonine phosphatases—for example, protein phosphatase-1 and -2A (PP1 and PP2A)—may also be susceptible to oxidative inactivation.67,68 Importantly, oxidized cysteine residues can be rescued by the actions of enzymes such as thioredoxin and glutaredoxin, suggesting that transient inactivation of phosphatases by H2O2 may have a functional role. Indeed, it has been proposed that transient phosphatase inhibition may be necessary for efficient transmission of signals via kinase cascades.64
Functions of ROS in normal hematopoiesis
The roles of ROS in normal hematopoiesis are a growing area in research. Indeed, there is accumulating evidence from murine and human HSC models that ROS management is critical for primitive hematopoietic cells. Specifically, elevated ROS appears to drive HSCs out of quiescence and reduces self-renewal capacity, resulting in rapid bone marrow failure if not remedied.69-71 Furthermore, the majority of the evidence suggests that v-Akt murine thymoma viral oncogene homolog (Akt) signaling via forkhead box-O (FOXO) family of transcription factors (which are important for maintaining antioxidant defense) plays a crucial role in maintaining HSC pools. For example, using an in vitro model, Rizo et al demonstrated that both normal and leukemic human CD34+ cells deficient for the polycomb family protein BMI1 showed reduced long-term culture capacity, suggesting that BMI1 plays a role in self-renewal of HSCs and progenitor cells. Importantly, the loss of self-renewal capacity coincided with a reduction of FOXO3A expression and an increase in intracellular ROS, and treatment with the antioxidant, N-acetylcysteine, partially rescued the phenotype.69 A similar effect was observed in mice deficient for Atm (which is directly downstream of Foxo3a). These mice developed a rapidly fatal bone marrow defect, which was again attributed to elevated ROS and reduced quiescence in the HSC and progenitor cell population.71 Furthermore, Tothova et al showed that conditional deletion of all Foxo isoforms in mice resulted in rapid bone marrow failure, because of exhaustion of Foxo-deficient HSCs. Foxo-deficient HSCs from these mice were less quiescent, showed increased apoptosis, and showed higher levels of intracellular ROS. Importantly, antioxidant treatment was sufficient to rescue this phenotype, suggesting that elevated ROS was an important factor in the increased HSC proliferation and loss of self-renewal observed.70
Studies that have directly targeted Akt expression also support the notion that this pathway is important in regulating quiescence in HSCs, and that ROS levels may have a significant influence. For example, Juntilla et al demonstrated that deficiency in both Akt1 and Akt2 resulted in poor HSC function.72 Interestingly, these HSCs showed the reciprocal phenotype to that seen by Tothova et al; Akt deficiency resulted in decreased intracellular ROS together with an increase in quiescence. However, no differences in Foxo mRNA were detected in Akt-deficient HSCs in this study, suggesting that the observed decrease in ROS was Foxo-independent.72 Conversely, Kharas et al showed that constitutively active Akt (myr-Akt) leads to loss of HSCs and bone marrow failure because of reduced quiescence and self-renewal in a similar manner to Foxo-deficient mice,73 supporting the notion that Akt signaling is important HSC maintenance. However, unlike the Foxo-deficient mice, this group did not detect increased ROS, suggesting that HSC loss in this model occurred via a ROS-independent mechanism.
Taken together, these studies suggest that ROS levels must be managed to maintain HSC quiescence. Indeed it appears that an “ideal” (and relatively low) level of intracellular ROS exists in normal HSCs, and controlled fluctuations in ROS levels may govern the self-renewal and proliferative capacity of these cells. Unregulated increases (or decreases) in ROS lead to significant alterations in HSC function. This sensitivity of HSCs to high levels of ROS may partly explain the observation that HSC niches tend to be located in the most hypoxic regions of the bone marrow.74,75 Furthermore, HSCs appear to have adapted their metabolic activity to suit this hypoxic environment,76 and in so doing may avoid increased mitochondrial ROS production that may otherwise occur under hypoxic conditions. Indeed, it appears that stem cells from other tissues appear to exhibit a similar preference for lower ROS levels; for example, mammary epithelial stem cells show lower levels of ROS than their more differentiated counterparts.77 The need to maintain a low level of ROS in HSCs to preserve HSC function contrasts with evidence from more mature myeloid cells, where it appears that an increase in intracellular ROS accompanies normal myeloid development in both mammalian and insect models.70,75,78,79
ROS also appear to influence other functions of HSCs in addition to self-renewal. Using a novel in vivo imaging technique, Lewandowski et al demonstrated that ROS-dependent expression of vascular cell adhesion protein 1 (VCAM-1) on bone marrow endothelial cells is required for the early stages of HSC bone marrow homing and localization in the context of transplantation.80 ROS may also be required for hematopoietic progenitor cell migration; murine c-Kit+ Lin− bone marrow cells deficient for Nox2 showed impaired chemotaxis, invasion, and actin reorganization in response to stromal cell–derived factor-1 (SDF-1). Interestingly, this phenotype was associated with reduced Akt phosphorylation.81 The requirement for ROS in hematopoietic cell migration has been reinforced by a recent study that showed that NAC treatment could suppress mobilization of c-Kit+ Lin− Sca-1+ cells in mice treated with G-CSF.82 ROS production is also closely associated with hematopoietic growth factor signaling. For example, NOX-derived ROS production has been implicated in granulocyte colony stimulating factor (G-CSF) signaling, where it contributes to cell-cycle progression,62 while a separate study demonstrated that stimulation of human hematopoietic cells with either granulocyte/macrophage(GM)–CSF, interleukin-3 (IL-3), stem cell factor (SCF), or thrombopoietin (TPO) triggered ROS production that played an important role in transduction of these growth factors' signals.61
Some of these studies have concluded that NOX oxidases are the major source of ROS that is implicated in processes such as cell migration81 and growth factor signaling.61,62 However, the sources of ROS that lead to bone marrow failure (eg, in mice deficient for Foxo70 ) are not yet clear. Indeed, neither mitochondrial ROS nor NOX oxidases have been excluded as the source of ROS in these models. It may be that bone marrow failure in these models is the result of failure to control accumulation of ROS generated at low levels from either the mitochondria, NOX oxidases, or both, or metabolic pathways. Certainly, in the case of Foxo-deficient mice,70 several Foxo targets encoding antioxidant proteins (eg, catalase and SOD) were found to be down-regulated, which may explain the ROS accumulation. However, it appears that Foxo activity cannot explain ROS accumulation in all cases72 ; therefore, more research is needed to establish how and why ROS accumulates in these models.
ROS production in myeloid disease
Evidence of elevated ROS has been detected in a variety of diverse pathologic states, including atherosclerosis, rheumatoid arthritis, and amyotrophic lateral sclerosis,83 and also several cancers, including chronic and acute myeloid malignancies, and also in Fanconi anemia (FA), a congenital disorder of hematopoiesis that strongly predisposes to specific solid tumors and myeloid leukemias.84 In the case of CML, ectopic expression of BCR-ABL alone has been shown to induce ROS production in hematopoietic cells,9,85 and it appears that ROS generation is critical for transformation by BCR-ABL.86 In addition, elevated oxidative stress markers such as malondialdehyde and protein-carbonyls were detected in the plasma of CML patients compared with matched controls.44,45 The degree of oxidative stress appears to increase during the accelerated phase of CML,45 whereas nonenzymatic antioxidants in the plasma were simultaneously depleted.87 Furthermore, individuals with a polymorphism leading to reduced activity of glutathione S-transferase π (GSTP1, an enzyme important in detoxifying by-products of DNA oxidation) showed a significantly increased risk of developing CML and were more likely to have a poorer prognosis.88 The sources of elevated ROS in CML patients remains unclear; however, in model systems both increased NOX4 activity9 and increased mitochondrial ROS85 have been cited.
Oxidative damage is also frequently detected in MDS patients, beginning with an early study published in the late 1990s that demonstrated the presence of oxidative DNA damage in CD34+ cells from MDS patients.89 These data were supported by subsequent studies in which expression of several antioxidant enzymes was increased in granulocytes from MDS patients,90 and oxidized purine and pyrimidine bases were more prevalent in CD34+ cells from MDS patient bone marrow than in controls.91 Iron overload is thought to be a major cause of oxidative stress in MDS patients, primarily as a result of the regular transfusions received by these patients as part of their treatment. Subsequently, free iron can promote oxidative stress in MDS patients possibly through generation of ROS via Fenton chemistry.92,93 Indeed, it appears that iron-chelation therapy in MDS patients with iron overload can be beneficial,92,93 although it is not yet clear how much of this benefit is because of decreased oxidative stress.94 Mutations in mitochondrial DNA are commonly detected in MDS patients95 and such mutations are associated with poor ETC function and elevated ROS96 ; however, it is not currently known whether this mechanism of ROS production significantly contributes to oxidative damage in these patients.
Unlike CML and MDS, to date it appears that oxidative stress has not been thoroughly examined in the closely related myeloproliferative neoplasm (MPN)/MDS disorders, such as juvenile myelomonocytic leukemia (JMML) and chronic myelomonocytic leukemia (CMML). Indeed, given the prevalence of mutations in the Ras pathway in these disorders,97 increased ROS production would be expected (see next paragraph), but this remains to be established.
There is also evidence of increased ROS and oxidative stress in models of AML and also in primary AML samples. Elevated ROS production and oxidative DNA damage were observed in a murine model of AML driven by expression of mutant N-Ras and B-cell CLL/lymphoma-2 (Bcl-2),98 while work within our group demonstrated that ectopic expression of activated Ras also elicits ROS production in human CD34+ cells.17 In patient studies, ROS production by resting and stimulated leukocytes was increased in samples from AML patients compared with leukocytes from healthy individuals,99,100 whereas another study detected an increased expression of oxidative stress response genes in AML patient samples.90 More recently, an increase in several oxidative stress markers was detected in relapsed AML patients compared with samples obtained at first diagnosis.16 A recent retroviral screen searching for novel genes associated with AML revealed that the gene encoding thioredoxin (TRX)–interacting protein (TXNIP) was a common retrovirus insertion site in mice that developed AML.101 The same study also demonstrated that TXNIP is also commonly overexpressed in AML patients. Considering that TXNIP binds to and inactivates TRX (an important protein in antioxidant defense),102 the association of TXNIP expression with AML in mice (and in patients) may in part be because of TXNIP-induced oxidative stress. The sources of increased ROS in AML patients are not well documented; however, our previous research suggested that human CD34+ cells expressing mutant Ras generate excess ROS via activation of NOX2.17 Ras mutations are among the most common molecular abnormalities detected in AML, and are also closely related with increased ROS production primarily through activation of NOX oxidases in a variety of other cancers.103-105
There is considerable evidence suggesting that oxidative stress is a key factor in many of the symptoms of FA. FA is a genetic hematologic disorder characterized by early-onset pancytopenia progressing to bone marrow failure.84 FA patients also exhibit various endocrinopathies (including type II diabetes mellitus), developmental defects, and skin hyperpigmentation. Importantly, FA patients frequently go on to develop MDS or AML. These patients fall into at least 13 complementation groups defined by the presence of inactivating mutations in FA proteins, which together function in a distinct pathway responsible for DNA repair. Evidence that oxidative stress plays a role in this disorder comes from several observations including elevated 8-oxoguanine and oxidized glutathione in FA patients,106 whereas antioxidant treatment or hypoxia reduces apoptotic propensity and chromosomal instability.107-109 Indeed it has been argued in a recent review that although defective DNA damage repair explains some FA features (eg, propensity to solid tumors and myeloid leukemia), FA-associated oxidative stress may be a more fundamental defect that better explains the entire spectrum of symptoms exhibited by FA patients.110 It appears that loss of some FA proteins may directly result in oxidative stress; recent evidence showed that FANCD2 directly recruits FOXO3A to the nucleus under conditions of ROS stress resulting in FOXO3A-dependent transcription of several antioxidant genes. Critically, this interaction was abolished in FANCA- or FANCD2-deficient cells.111
Finally, disturbances in metabolic processes that are inherent in leukemic cells can also up-regulate ROS. Specifically, it has recently been demonstrated that mutations in isocitrate dehydrogenase 1 (IDH1) or IDH2 are frequent in AML but scarce in most other cancers.112 Mutant IDH exhibits a significant shift in activity, favoring conversion of isocitrate to 2-hydroxyglutarate (2-HG) instead of α-ketoglutarate (α-KG).113 This shift in activity has several knock-on effects, the most relevant being an increase in intracellular ROS, apparently mediated by 2-HG itself.114 It might also be expected that changes in metabolism and proliferative rate would affect mitochondrial ROS output in leukemic blasts, but this does not appear to have been formally investigated.
Consequences of increased ROS in myeloid disease
The consequences of elevated ROS in myeloid leukemia fall into 2 major categories—the first are the results of nonspecific oxidative damage to biomolecules, the second are more specific effects arising from hyperactivation of ROS signaling pathways.
Nonspecific oxidative damage occurs when elevated ROS levels persist and the cell's antioxidant defenses become exhausted. This leads to lipid peroxidation, increased intracellular pH, and, most significantly, oxidative DNA damage leading to single- and double-stranded DNA breaks via direct chemical reaction, or indirectly via activation of carcinogens.115 The establishment of such an environment is extremely favorable for tumorigenesis, and promotes selection of those clones that are able to survive and flourish. Recent evidence suggests that such a process may drive disease progression in AML; patients with an internal tandem duplication of fms-like tyrosine kinase-3 (Flt3-ITD) exhibit elevated NOX-derived ROS and increased double-stranded DNA breaks, which may partly explain the poorer prognosis of Flt3-ITD+ patients116 ; indeed, the role of ROS in promotion of genomic instability and progression of myeloid disease has been the subject of a recent review.7 In the normal state, most cells respond to potentially genotoxic stress by induction of cell-cycle arrest and/or apoptosis, in an attempt to preserve genomic stability. This response is mediated by stress pathways downstream of stress-activated protein kinases (SAPKs) such as c-Jun N-terminal kinase (JNK) and p38MAPK, which become phosphorylated in response to genotoxic stress and subsequently promote cell-cycle arrest through INK4 protein family members and TP53.117,118 In particular, p38MAPK plays a critical role in orchestrating the response to ROS stress.119,120 Dolado et al showed that murine embryonic fibroblasts (MEFs) expressing oncogenic H-Ras were dependent on functional p38MAPK to avoid transformation, and that p38MAPK specifically responded to elevated ROS induced by this oncogene, possibly via NOX1 and 4.120 In the same study, several cancer cell lines were shown to have specifically silenced activation of p38MAPK in response to ROS, whereas activation of p38MAPK in response to ultraviolet radiation was normal, suggesting that the specific ability to tolerate higher levels of ROS was beneficial to the malignant clones. p38MAPK was also shown to specifically respond to elevated ROS in murine Lin− Sca-1+ c-Kit+ hematopoietic cells (arising from treatment with butathionine sulphoximine [BSO] or ataxia telangiectasia-mutated [Atm] deficiency).119 Interestingly, inactivation of downstream targets of p38MAPK such as p15INK4B and p16INK4A is frequently detected in AML,121-123 suggesting that evasion of stress responses (including ROS stress responses) may be important in this disease.
Increased ROS may also manifest its effects in myeloid disease by aberrantly altering signaling in pathways that are ROS-regulated, particularly pro-proliferative and/or survival pathways. For example, in a CML model, expression of the pro-proliferative molecule Fyn was shown to be dependent on BCR-ABL–induced ROS, which in turn activated the transcription factors specificity protein 1 (Sp1) and early growth response protein 1 (Egr1).124 Studies examining models of AML have come to similar conclusions. For example, expression of the RUNX1-ETO fusion protein in Drosophila melanogaster promoted proliferation of primitive hematopoietic cells in an ROS-dependent manner.125 Studies investigating the effect of IL-3 on the M07e human AML cell line noted that the prosurvival effect of this cytokine was mediated by NOX2-derived ROS production, and IL-3–promoted survival was suppressed by NOX inhibitors or specific knockdown of NOX2 expression.8,126 Furthermore, a very recent study has concluded that NOX-derived ROS contributes to growth and migration of hematopoietic cells expressing a variety of oncogenic tyrosine kinases.18 The molecular targets of ROS that mediate these prosurvival or pro-proliferative effects in malignant cells are not currently known. However, given that ROS are already known to mediate prosurvival and pro-proliferative signals in normal cells,58-62 it seems likely that similar (albeit hyperactive) mechanisms are operating in malignant cells that overproduce ROS. In particular, there is evidence that phosphorylation of Akt driven by ROS mediates the prosurvival effects of several growth factors,60,62 but more research is required to establish whether ROS production by malignant cells promotes survival via a similar mechanism.
Finally, research from our own group suggests that NOX2-derived ROS production in human CD34+ cells drives cell-cycle progression, but interestingly, this effect was observed only in the context of constitutively active Ras; the proliferation of normal cells was actually hindered by the presence of excess ROS.17 These context-dependent effects of ROS have implications for in vivo disease. ROS such as H2O2 generated by malignant cells may confer a proliferative advantage on the malignant population, but may also freely diffuse into neighboring normal cells, hindering normal cell proliferation in a paracrine manner. If such an environment persisted in the bone marrow, excess H2O2 generated by malignant cells could potentially disturb normal hematopoiesis. Indeed, this notion is supported by evidence that cells within the nonmalignant population within AML patients show signs of oxidative stress.90 This situation could be further exacerbated by the fact that phosphatase inhibition by ROS can establish a positive feedback loop, where promoters of NOX oxidase activity become hyperphosphorylated and prolong NOX activation.127
Is there a case for targeting ROS in myeloid disease?
Cytotoxic drugs currently used in standard leukemia therapy almost exclusively attack the DNA replication process within malignant cells, with the rationale that cancer cells are selectively sensitive to these agents because of their increased proliferation. However, targeting tumor cells on the basis of increased DNA replication has its limitations. Nonmalignant dividing cells are also targeted by these therapies and the resulting toxicity precludes treatment of some patients and forces premature termination of treatment in others.128 More importantly, recent evidence suggests that many tumors harbor rare quiescent clones that are resistant to these standard chemotherapies and may be responsible for disease relapse.129,130 For this reason, novel therapies that target the specific molecular or metabolic abnormalities within a leukemic cell are highly sought. Indeed, the success of several compounds justifies this approach to treatment, for example, imatinib and other specific tyrosine kinase inhibitors in CML,131 and all-trans retinoic acid (ATRA) in acute promyelocytic leukemia (APL).132
In a similar manner, ROS production by malignant cells may represent a novel potential therapeutic target, considering that ROS levels in malignant cells are frequently higher than their normal cellular counterparts. However, there are 2 possible approaches to manipulating ROS in malignant cells for therapeutic gain. The first (referred to herein as the pro-oxidant approach) involves amplification of existing ROS stress in malignant cells by treating them with pro-oxidants, that is, compounds that introduce exogenous ROS or promote its production.15 Such treatment is expected to cause catastrophic chemical damage only in those cells where oxidative stress was preexistent. Interestingly, it has been suggested that some current mainstay cancer therapies may rely on this principle to exert their full cytotoxic effect,133,134 in that the highly stressed state of malignant cells may partly explain the selectivity of these cytotoxic drugs.135 A similar effect may be achieved by inhibiting intracellular antioxidants, and there is evidence that molecules with antioxidant capacity such as GSH136 and heme oxygenase-1 (HO-1)137 are augmented in cancer cells (possibly in response to increased oxidative stress) and that this increased antioxidant capacity may play a role in drug resistance.136 Indeed, several compounds with pro-oxidant capacity appear to be effective against both leukemic cell lines and primary leukemic blasts. For example, arsenic trioxide (ATO) is a molecule that is currently approved for treatment of relapsed APL and has pro-oxidant properties. The mechanism of ROS generation by ATO is not entirely clear, but may occur by thioredoxin inhibition,138 augmentation of mitochondrial ROS,139 or NOX activation.140 Regardless of the source of ROS, recent research suggests that ATO-induced ROS production plays a critical role in degradation of PML-RARα fusion proteins in ATO-treated APL cells.141 Other pro-oxidant compounds are attracting interest as antileukemic agents,142,143 such as isothiocyanates and adaphostine. Isothiocyanates act by depleting GSH pools, and efficiently kill fludarabine-resistant chronic lymphocytic leukemia (CLL) cells144 and imatinib-resistant CML cells,145 while showing little toxicity to normal hematopoietic cells. Isothiocyanates have also been shown to synergize with ATO in killing CML- and AML-derived cell lines in vitro.146 Similarly, adaphostin is a tyrphostin kinase inhibitor that is able to induce apoptosis in a variety of BCR-ABL–expressing CML cells, including those expressing imatinib-resistant isoforms of BCR-ABL, and this appears to be primarily via up-regulation of ROS and subsequent DNA damage–induced apoptosis.147
The second approach to targeting ROS in cancer is to use antioxidant molecules to suppress the high levels of ROS observed in some cancer cells. The rationale behind this approach is that ROS-generating cancers may develop a degree of “ROS addiction,” supported by evidence that NOX4-derived ROS promotes survival in some pancreatic cancers.148 In addition, it has been argued that antioxidant treatment may alleviate chemotherapy-related toxicity, reducing the requirement for dose reduction in some patients and allowing an increased proportion of patients to complete their therapy.149 Unlike the pro-oxidant approach, the effects of reducing ROS using antioxidant therapy has not been extensively investigated in leukemias, however, patients with low- to intermediate-risk MDS did appear to benefit from treatment with the aminothiol prodrug, amifostine.150 Another study demonstrated a trend of longer clinical progression-free survival and overall survival in CML patients when they were treated with vitamin A in combination with standard chemotherapy, although this trend was not significant.151 A recent review conducted by Block et al examined the effect of combined antioxidant/chemotherapy treatment in patients with a variety of cancers in 19 randomized clinical trials. Interestingly, 17 of the 19 trials examined showed either nonsignificant or significant advantages in terms of treatment response and/or overall survival in patients who received antioxidant supplements.152 Finally, because oxidative stress may contribute to genomic instability and myeloid disease progression,7 antioxidant treatment may serve to reduce DNA damage and potentially slow disease progression. Indeed, a recent study demonstrated that relapse in AML correlates with an escalation of oxidative stress16 ; however, the effect of antioxidant treatment on AML progression has not yet been examined.
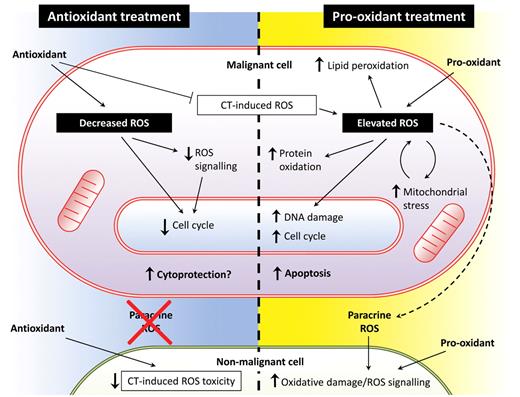
In answer to the question posed at the beginning of this section, it does appear that both pro-oxidant and antioxidant approaches have merit, but more evidence is needed that specifically relates to myeloid leukemias, particularly using the antioxidant approach. There are also some potential disadvantages of each approach (Figure 2). For example, as discussed previously, pro-oxidant strategies appear to be effective in vitro (and in vivo in the case of ATO in APL132 ); however, such strategies must contend with the potential collateral damage to normal cells that could be caused if pro-oxidant agents are introduced into patients. On the other hand, critics of the antioxidant approach argue that ROS depletion potentially conflicts with the pro-oxidant effects of mainstay agents. Although the study by Block et al may go some way to allay these concerns,152 more research is required to establish whether antioxidants are cytoprotective to cancer cells.
Antioxidant treatment versus pro-oxidant treatment as a therapy for hematologic malignancy. ROS production in myeloid leukemia cells may present an exploitable therapeutic target. Both antioxidant and pro-oxidant strategies may be effective, but both have potential advantages and disadvantages. The effects of antioxidant treatment on malignant cells (left side of figure) are likely to include reduced proliferative drive, which may reduce tumor burden and also protect nonmalignant cells from oxidative damage, particularly when administered in combination with chemotherapeutic (CT) agents. However, there are also concerns that suppression of cell cycle and antagonism of chemotherapy-induced ROS may adversely affect treatment efficacy. Pro-oxidant treatment (right side of figure) induces further ROS beyond that already produced by the malignant cell, either by depleting antioxidant defenses or augmenting ROS production. Treatment-induced oxidative stress combined with the intrinsic stress already present in the malignant cell leads to lipid peroxidation, oxidation of redox-sensitive residues within proteins, and DNA oxidation resulting in base-transversion and DSBs. Furthermore, damage to the electron transport chain and mutations in mitochondrial DNA can lead to a cycle of increased mitochondrial ROS. Elevated ROS has also been shown to contribute to cell-cycle progression in some contexts, which may increase tumor burden, but may simultaneously sensitize malignant cells to mainstay treatments. Although these factors may lead to induction of apoptosis in malignant cells, elevated ROS will increase the DNA mutation rate in any cells that fail to undergo apoptosis, possibly leading to selection of resistant clones. Furthermore, ROS generated by malignant cells may have paracrine effects on ROS signaling and oxidative damage in nonmalignant cells, and this effect would be augmented during treatment with pro-oxidants.
Antioxidant treatment versus pro-oxidant treatment as a therapy for hematologic malignancy. ROS production in myeloid leukemia cells may present an exploitable therapeutic target. Both antioxidant and pro-oxidant strategies may be effective, but both have potential advantages and disadvantages. The effects of antioxidant treatment on malignant cells (left side of figure) are likely to include reduced proliferative drive, which may reduce tumor burden and also protect nonmalignant cells from oxidative damage, particularly when administered in combination with chemotherapeutic (CT) agents. However, there are also concerns that suppression of cell cycle and antagonism of chemotherapy-induced ROS may adversely affect treatment efficacy. Pro-oxidant treatment (right side of figure) induces further ROS beyond that already produced by the malignant cell, either by depleting antioxidant defenses or augmenting ROS production. Treatment-induced oxidative stress combined with the intrinsic stress already present in the malignant cell leads to lipid peroxidation, oxidation of redox-sensitive residues within proteins, and DNA oxidation resulting in base-transversion and DSBs. Furthermore, damage to the electron transport chain and mutations in mitochondrial DNA can lead to a cycle of increased mitochondrial ROS. Elevated ROS has also been shown to contribute to cell-cycle progression in some contexts, which may increase tumor burden, but may simultaneously sensitize malignant cells to mainstay treatments. Although these factors may lead to induction of apoptosis in malignant cells, elevated ROS will increase the DNA mutation rate in any cells that fail to undergo apoptosis, possibly leading to selection of resistant clones. Furthermore, ROS generated by malignant cells may have paracrine effects on ROS signaling and oxidative damage in nonmalignant cells, and this effect would be augmented during treatment with pro-oxidants.
Summary and perspective
Despite the progress made in the past 40 years, myeloid leukemias continue to be difficult to treat. Current therapeutic strategies appear to have reached the limit of their effectiveness; novel therapeutic strategies that exclusively target malignant cells are required. Increased oxidative stress in some myeloid leukemias may indeed represent a therapeutic target, and early encouraging results from in vitro studies and clinical trials suggest that ROS modulation therapy in myeloid leukemia patients warrants further investigation. Indeed, either pro-oxidant or antioxidant approaches appear to be beneficial, and it may be that certain myeloid leukemias (or myeloid leukemia subtypes) respond preferentially to one particular approach over the other. However, it is clear that we require a better understanding of the roles and prognostic impact of elevated ROS in myeloid leukemias before we can construct effective ROS-based therapies. A critical requirement for future studies will be to focus on identifying the source and species of ROS generated by leukemic cells. Furthermore, it is clear that ROS must be constitutively produced for normal cell function, but it is unclear where the boundary lies between physiologic and pathophysiologic levels of ROS. Finally, it is known that several chemotherapeutic agents rely partly or wholly on an ROS-based mechanism of action and it may be interesting to see whether the effectiveness of these agents is dependent on basal cell ROS production by the malignant cell population. Currently, our research focuses on measuring ROS production in primary AML samples, and understanding the role that ROS may play in AML pathogenesis and response to treatment.
Acknowledgments
The authors thank Dr Steve Knapper (Cardiff University) for critically appraising the manuscript.
This work was supported partly by the Medical Research Council (United Kingdom) and by Leukaemia and Lymphoma Research.
Authorship
Contribution: All authors wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alex Tonks, Department of Medical Genetics, Haematology and Pathology, School of Medicine, Cardiff University, Cardiff, United Kingdom CF14 4XN; e-mail: tonksa@cf.ac.uk.