It has been 40 years since Charlotte Friend demonstrated the unbelievable for the second time.
Friend, a scientist on faculty at the Mt Sinai School of Medicine in New York, had become renowned for her highly controversial observation that a virus (the Friend Leukemia Virus; FLV) could cause erythroleukemia. Now she had a second, perhaps even more stunning find—an observation that would take her full circle. While studying a thick suspension of her ivory-white erythroleukemia cells, she entered the laboratory one morning to find the inexplicable. Overnight, the suspension of leukocytes she stored the evening before had turned into a mass of vibrant red cells. In an effort to preserve her cells, she had incubated them with a routine solvent called DMSO (dimethyl sulfoxide). She deducted with time that the DMSO must have pushed the immature cells from their undifferentiated hemoglobin free state to a mass of terminally differentiated red blood cells, making them now full-fledged erythrocytes.1
Her groundbreaking observation that a small molecule could induce the differentiation of a malignant cell to a normal one was nearly as controversial as her claim a decade earlier. It took 25 years more before the steps induced by DMSO in Friend's erythroleukemia model would be elucidated. DMSO induced all the obvious events one would expect to see in a primitive erythroid precursor on its way to becoming an erythrocyte. The accumulation of globin messenger RNA, increased iron assimilation and heme biosynthesis, accumulation of hemoglobin, the emergence of erythrocyte membrane proteins, cell-cycle arrest, and accumulation of hyperacetylated histone.
In the years following her 1971 publication, attention quickly shifted to the finding that accumulation of hyperacetylated histone seemed to be the biologic event that defined those agents capable of inducing differentiation. It was not until 1996 when Schreiber and colleagues2 developed an assay to isolate the presumptive enzymes that were the target for DMSO. His biochemical strategy for pulling down HDACs from within the cell helped the field to establish HDACs as the substrate for those compounds that induced accumulation of hyperacetylated histone.
The relatively high concentrations of DMSO (high millimolar) required to induce histone acetylation led chemists like Breslow at Columbia University to synthesize better inhibitors. Their efforts led to the synthesis of the more potent hexamethylene bisacetamide (HMBA), first reported by Marks and colleagues in 1976.3 While HMBA produced signals of clinical activity in patients with solid tumors, the associated thrombocytopenia curtailed the drugs development. Repeated rounds of synthesis led Breslow to suberoylanilide hydroxamic acid, more affably known as SAHA. SAHA, more than 2500 times more potent than HMBA, would become the first HDAC inhibitor approved for the treatment of cancer.4
Nearly coincident with the evolving SAHA development, FRK228 was being studied by the National Cancer Institute (NCI) in a phase 1 trial. The last patient treated on study was a patient with refractory peripheral T-cell lymphoma (PTCL). This patient experienced a complete response and remains alive 10 years later. The trial was expanded to include additional patients with PTCL, confirming a substantial signal in T-cell malignancies. In nearly parallel development paths, both of these unique HDAC inhibitors found their niche. Vorinostat attained approval in 2006, depsipeptide in 2009, both in CTCL. The experience unequovically established, for unclear reasons, that targeting HDAC is a valid therapeutic strategy for these challenging diseases.
In this issue of Blood, Piekarz et al report on their phase 2 experience with depsipeptide in patients with PTCL.5 They treated 47 patients, most of whom (57%) had PTCL–not otherwise specified or angioimmunoblastic lymphoma (15%). As noted with HMBA and SAHA, thrombocytopenia was one of the most common hematologic toxicities, with approximately 15% of patients experiencing grade 3 toxicity (no grade 4 noted). Similar to other studies with HDAC inhibitors, nearly 40% of patients experienced fatigue, and 20% experienced some anorexia, although none reaching grade 3 or 4. The toxicity that has garnished the most attention with depsipeptide has been the ECG changes. In this report, 64% of patients experienced a grade 1 or 2 event.5 A detailed review of the unexplained cardiac deaths on study revealed that most patients who experienced an event also had established risk factors for sudden death. Based on these data, rigorous K+ and Mg+ management is required to receive the drug. The overall response rate among these patients was 38%, including 18% complete response who experienced very durable remissions.
In the nearly 4 decades since Friend demonstrated the effects of DMSO in her models of erythroleukemia, a completely new era of cancer biology and therapeutics has emerged. Understanding how epigenetic influences contribute to the malignant phenotype has now become one of the most exciting areas in all of cancer medicine. Furthermore, the ability to pharmacologically modulate this biology with small molecules that inhibit HDAC or lead to promoter hypomethylation has created new treatment platforms for a host of diseases.
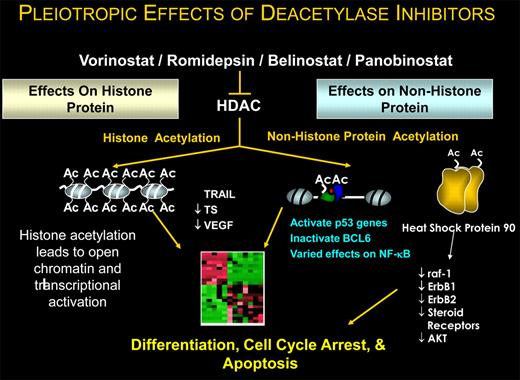
What remains curious about HDAC inhibitors is understanding how they work in any cancer (see figure). Preclinical data have shown these drugs induce not merely differentiation but also cell-cycle arrest and apoptosis. While we repeatedly refer to these drugs as HDAC inhibitors, it is now clear that histone is hardly the only, let alone the most important, protein substrate affected. Scientifically, the focus has shifted to understanding how these agents modify the posttranslational state of specific oncogenes and tumor suppressor genes. Pharmacologically modulating the activity of these regulatory proteins will have important therapeutic ramifications.
While there are now many drugs with activity in PTCL, it is apparent that the HDAC inhibitors may have a universal class effect. It is hard to imagine, now 40 years later, that Friend could have envisioned the impact her serendipitous experience might have in CTCL/PTCL. Patients with these diseases, at long last, have a plethora of friends. The ultimate irony is that the disease where Friend's observations had their impact happens to be the disease that claimed her life. After a courageous 6-year battle, she died of lymphoma in 1987. With all the outstanding achievements we are making in cancer on an almost daily basis, its easy to forget how it all began.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal