To the editor:
We would like to complement our recently reported data on the detection of the Merkel cell polyomavirus (MCPyV) in chronic lymphocytic leukemia (CLL) cells.1 We have shown that MCPyV could be identified by PCR in highly purified CLL cells of 19 of 70 (27.1%) CLL patients. In 6 cases, a 246 bp deletion in the helicase region of the large T-antigen (LTAg) of MCPyV was found. Truncating mutations or deletions in the LTAg of MCPyV lead to a loss of the helicase activity and thus prevent viral replication.2 In addition, in 2 of these CLL cases deleted LTAg was detected concomitant to wild-type MCPyV.
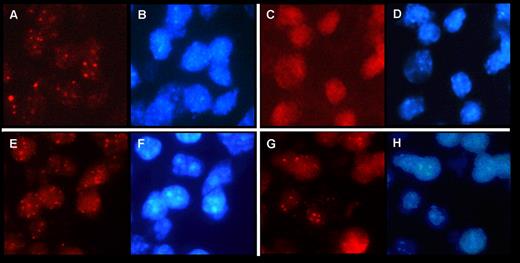
Here we confirm the presence of the MCPyV genome in CLL cells by using fluorescence in situ hybridization (FISH). MCPyV-FISH was performed according to a recently published FISH protocol3 to detect human papilloma virus (HPV) in formalin-fixed and paraffin-embedded (FFPE) tissue. The MCPyV DNA probe we used was isolated from a Merkel cell carcinoma (MCC) and contained a 4 bp deletion creating a stop codon before the helicase domain. FISH was applied to all 6 FFPE bone marrow trephines (EDTA decalcified) in which we previously detected the 246 bp LTAg MCPyV deletion. Three MCPyV DNA-positive MCCs, as detected by PCR,4 were used as positive controls showing punctate nuclear signals in MCC indicative for integrated MCPyV in the tumor cells (Figure 1A). Negative controls included omission of the MCPyV probe and several MCPyV-negative tissues, that is, a mantle cell lymphoma and 2 colon carcinomas. None of the negative controls revealed a FISH signal (Figure 1C). Of the 6 CLL cases harboring the 246 bp deletion in the MCPyV-LTAg, 4 revealed punctate signals in the nucleus (Figure 1E,G), comparable to those observed in MCC. The observation that nuclei sometimes show FISH signals differing in intensity (granular staining pattern) might suggest that in addition to MCPyV-DNA integration, transcribed viral RNA is also present.3,5 FISH analysis on the remaining 2 MCPyV-positive CLLs were inconclusive, most likely because of tissue overfixation and/or poor DNA quality.
MCPyV detection by FISH in previously reported MCC and CLL cases.1,4 MCPyV FISH was carried out using tyramide signal amplification (TSA) as described previously.3,5 Signal evaluation was performed with a Leica DM 5000 B fluorescence microscope (Leica) using 1000× magnification and specific filter sets for Texas red to visualize MCPyV (A,C,E,G) and DAPI showing blue nuclear counterstaining (B,D,F,H). (A-B) MCPyV detection in MCC and corresponding DAPI (B). (C-D) Negative control on MCC tissue without probe and corresponding DAPI (D). (E-F) MCPyV detection in CLL cells of a bone marrow trephine and corresponding DAPI (F). (G-H) MCPyV detection in another CLL case in a bone marrow trephine and corresponding DAPI (H).
MCPyV detection by FISH in previously reported MCC and CLL cases.1,4 MCPyV FISH was carried out using tyramide signal amplification (TSA) as described previously.3,5 Signal evaluation was performed with a Leica DM 5000 B fluorescence microscope (Leica) using 1000× magnification and specific filter sets for Texas red to visualize MCPyV (A,C,E,G) and DAPI showing blue nuclear counterstaining (B,D,F,H). (A-B) MCPyV detection in MCC and corresponding DAPI (B). (C-D) Negative control on MCC tissue without probe and corresponding DAPI (D). (E-F) MCPyV detection in CLL cells of a bone marrow trephine and corresponding DAPI (F). (G-H) MCPyV detection in another CLL case in a bone marrow trephine and corresponding DAPI (H).
In analogy to the punctate nuclear signals observed in anogenital and oropharyngeal lesions by HPV-FISH3,5 and in MCC by MCPyV-FISH, we have interpreted the present findings as integration of MCPyV in CLL cells. This finding additionally strongly supports a possible involvement of MCPyV in a significant subset of CLL.
Authorship
Acknowledgments: We thank Annick Haesevoets and Andrea Ruland for excellent technical assistance.
Contribution: A.M.H. performed experiments and analyzed data; E.-J.M.S. analyzed data, contributed vital knowledge, and helped writing the paper; N.D.P. analyzed data and contributed tools; C.P., A.K.K., and H.M.K analyzed data, contributed material and knowledge, and helped write the paper; G.C. contributed vital new research tools for FISH; C.-M.W. analyzed data, contributed material and knowledge, and helped write the paper; A.z.H., the principal investigator, designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Axel zur Hausen, MD, PhD, Department of Pathology, Maastricht UMC, P. Debyelaan 25, 6229 HX, Maastricht, The Netherlands; e-mail: axel.zurhausen@mumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal