To the editor:
We read with interest the recent article by Feuchtinger and colleagues about cytomegalovirus (CMV) pp65-specific T-cell infusion in the treatment of drug-resistant (R) CMV infection in allograft recipients.1 Viral load was reduced or cleared in 15 patients (83%) without graft-versus-host disease (GVHD) induction. Despite the encouraging results reported in this and previous studies,1-6 the clinical use of CMV-specific T-cell adoptive immunotherapy (CMV-TC) has been limited to a few transplant centers where the transplantation volume and the incidence of CMV disease are high enough and the cell-processing facilities technically advanced enough to justify developing an adoptive antiviral immunotherapy program. The time taken to prepare patient-specific products and the lack of virus-specific T cells in cord blood and seronegative donors also restricts application. Furthermore, it is not known whether nonspecific unmanipulated donor lymphocyte infusions (U-DLI) may have clinical activity against R-CMV that is comparable with ex vivo–generated CMV-TCs.
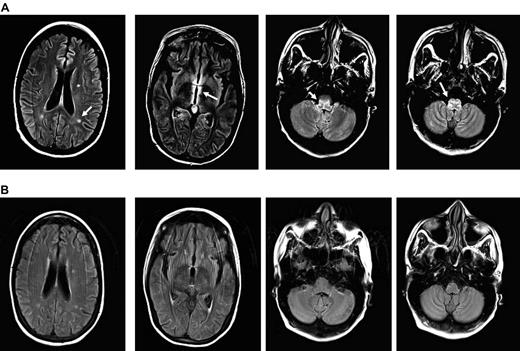
We recently treated a 29-year-old woman with R-CMV encephalitis with 5 sequential donor lymphocyte infusions at escalating doses using her backup U-DLI/stem cell products. The patient had received an A antigen-mismatch unrelated allogeneic peripheral blood stem cell transplant after myeloablative conditioning with total-body irradiation and melphalan for multiply relapsed γ-δ T-cell lymphoma. Both the patient and her donor were CMV antibody–reactive before transplantation. GVHD prophylaxis included tacrolimus, methotrexate, and antithymocyte globulin. She engrafted on day +18 and developed a presumed gut GVHD requiring a 2-week course of systemic corticosteroid. CMV reactivation, measured in plasma, was first noted by PCR on day +7 (2951 copies/mL) when she had also pneumonitis. She spent most of her posttransplantation period in the hospital because of persistent CMV viremia and recurrent bacteremia. Her viremia was never controlled despite prolonged courses of ganciclovir or foscarnet treatments. There was also no evidence of sustained CD4/CD8 recovery despite discontinuation of immunosuppression. Her lymphoma remained in remission with 100% donor myeloid and lymphoid chimerism by bone marrow and peripheral blood short-tandem repeat analyses. On day +107, she developed increasing somnolence, disorientation, nystagmus on a right gaze, and profound weakness. The diagnosis of CMV encephalitis was made based on MRI of the head, which showed restricted diffusion areas and round lesions within the periventricular white matter, medial left temporal lobe, and medulla with associated enhancement (Figure 1A), and high titers (388 660 copies/mL) of CMV detected in her cerebrospinal fluid (CSF) by quantitative PCR. CSF sample showed WBC of 2, RBC 0, normal glucose, and slightly elevated protein. Cytology revealed a few lymphocytes and monocytes. Simultaneous blood CMV viral load was 49 680 copies/mL. It was later found that the CMV was ganciclovir-resistant because of UL97 L595W mutation, detected in a blood sample. UL54 mutation was also tested by PCR but the result was inconclusive because of technical reasons. The patient also received leflunomide, cidofovir, IVIG, and CMV-specific IVIG at different time points for persistent CMV with producing marginal benefit, if any.
MRI images of CMV encephalitis before and after U-DLI. (A) Axial T2 fluid-attenuated inversion recovery (FLAIR) images demonstrate 3 new white matter lesions, increased signal, and edema in the periventricular region most notable around the third ventricle and medulla (white arrows). (B) Axial FLAIR images demonstrate resolution of previously described lesions and increased signal/edema 6 months after the initial U-DLI.
MRI images of CMV encephalitis before and after U-DLI. (A) Axial T2 fluid-attenuated inversion recovery (FLAIR) images demonstrate 3 new white matter lesions, increased signal, and edema in the periventricular region most notable around the third ventricle and medulla (white arrows). (B) Axial FLAIR images demonstrate resolution of previously described lesions and increased signal/edema 6 months after the initial U-DLI.
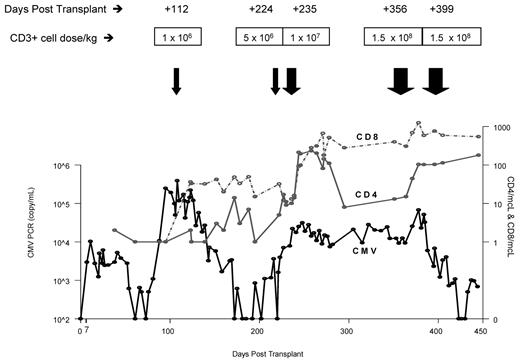
The patient's neurologic status and T-cell counts remained unchanged despite a decline in CMV copy number after the initial U-DLI, which was given on day +112 (Figure 2). She was 100% donor T-cell chimera before DLI. Following the third U-DLI, she developed localized chronic skin and ocular GVHD, which responded to topical treatments. This was associated with temporary increase in peripheral blood CD4/CD8 counts. After the fourth U-DLI, given at higher doses, her CD4/CD8 counts further increased and remained high afterward. This was clearly associated with decrease in her CMV viral load by PCR (Figure 2) and CMV antigenemia. No steroid therapy was given during any of the DLIs. Antiviral therapy was discontinued because of lack of clinical efficacy. Her neurologic recovery, however, lagged behind viral clearance following U-DLIs. She developed biopsy-proven grade 2 skin GVHD after the last (fifth) high-dose DLI, which responded to a short course of a systemic steroid. Most recently, her CMV was undetectable or positive at very low titer with relatively high peripheral T-cell counts. Her neurologic condition improved in parallel with resolution of MRI lesions (Figure 1B). A follow-up CSF sample examination on day +357 was negative for CMV by PCR.
Trend of CMV viral load over time in relation to peripheral blood CD4 and CD8 counts. The viral load less than 500 copies/mL is reported CMV-negative by PCR. CMV antigenemia levels also declined over time in parallel to the decline in CMV viral copy numbers.
Trend of CMV viral load over time in relation to peripheral blood CD4 and CD8 counts. The viral load less than 500 copies/mL is reported CMV-negative by PCR. CMV antigenemia levels also declined over time in parallel to the decline in CMV viral copy numbers.
Our observation of using previously collected and stored U-DLIs in 2 allogeneic HCT recipients, one with drug-resistant CMV encephalitis reported here and the other case with refractory aspergillus pneumonia7 suggests that sequential administration of escalating doses of U-DLI may have therapeutic value in treating opportunistic infections in allograft recipients. Without a comparative trial, it is difficult to determine whether ex vivo T-cell cloning and expansion is essential to induce CMV-specific immune responses in vivo. CMV-specific cytotoxic memory T cells that are present in donor lymphocyte product may be restimulated and expand after antigenic exposure in vivo. There is ample evidence to suggest that CMV reactivation is controlled by CMV-TCs.8,9 However, recent studies also suggest that natural killer cells play a role in protecting against CMV10,11 and U-DLI product could provide such additional cellular elements.
The patient's CD4 and CD8 counts remained severely suppressed despite discontinuation of immunosuppressive therapy. Prolonged immune-suppression has been partly attributed to the interactions between CMV and T-cell functions such as cytokine production and adhesion molecule expression.12 The CMV load dropped significantly after the fourth U-DLI. The initial antiviral effect was transient possibly because of the cell dose and the long interval between the first and second infusions. The last 2 infusions were given in closer proximity and contained relatively high CD3+ T-cell doses (1.5 × 108 CD3+ cells/kg). Significant increases in CD4/CD8 T-cell levels were demonstrated after each infusion.
Lymphocyte infusion may also be a considered to prevent CMV and perhaps other opportunistic infections in such high-risk patients by targeting predetermined CD4/CD8 counts. However, there is a potential risk of GVHD after multiple infusions especially when given at large doses. A trial is needed to determine benefits of preemptive DLI over the risk of GVHD in this setting. High-risk patients can be selected by using the recent Seattle data.13 Detection of CMV viremia in the setting of severe lymphopenia (< 50 at 3 months and < 300 after 3 months) and CMV-specific T-cell immunodeficiency were strong predictors of late CMV disease (18%) and death (46%) after allogeneic HCT.13
In summary, the clinical benefit observed in our case with refractory CMV encephalitis, which is almost always a fatal condition,14 may offer clinicians a potentially effective treatment option if CMV-specific T-cell therapy is not available.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gorgun Akpek, University of Maryland Greenebaum Cancer Center, 22 South Greene St, N9 E12, Baltimore, MD 21201-1595; e-mail: gakpek@umm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal