Abstract
Clinical manifestations of Plasmodium falciparum infection are induced by the asexual stages of the parasite that develop inside red blood cells (RBCs). Because splenic microcirculatory beds filter out altered RBCs, the spleen can innately clear subpopulations of infected or uninfected RBC modified during falciparum malaria. The spleen appears more protective against severe manifestations of malaria in naïve than in immune subjects. The spleen-specific pitting function accounts for a large fraction of parasite clearance in artemisinin-treated patients. RBC loss contributes to malarial anemia, a clinical form associated with subacute progression, frequent splenomegaly, and relatively low parasitemia. Stringent splenic clearance of ring-infected RBCs and uninfected, but parasite-altered, RBCs, may altogether exacerbate anemia and reduce the risks of severe complications associated with high parasite loads, such as cerebral malaria. The age of the patient directly influences the risk of severe manifestations. We hypothesize that coevolution resulting in increased splenic clearance of P. falciparum–altered RBCs in children favors the survival of the host and, ultimately, sustained parasite transmission. This analysis of the RBC–spleen dynamic interactions during P falciparum infection reflects both data and hypotheses, and provides a framework on which a more complete immunologic understanding of malaria pathogenesis may be elaborated.
Introduction
Malaria is an infectious, hematologic disease. Plasmodium falciparum infection—on which this review is focused—is one of the most frequent acquired red blood cell (RBC) disorders worldwide.1 During the asexual and sexual intraerythrocytic development of P falciparum, multiple molecular processes contribute to the remodeling of infected and uninfected RBCs,2,3 but how these modifications lead to disease is not entirely clear. The spleen, which filters out altered RBCs,4 is likely an important player in the broad spectrum of clinical manifestations of malaria, in particular, anemia. The aim of this review was to analyze relevant data and hypotheses on how splenic physiology may impact on malaria pathogenesis. Several factors of complexity were taken into consideration, including the dynamic, stage-dependent alterations of RBC induced by P falciparum,2 the diverse mechanisms by which the spleen senses subtle RBC alterations,4 and host and parasite factors that influence clinical outcome. We have focused on the corpuscular, RBC-based dimension of malaria pathogenesis in humans. An improved understanding would help select the best research approaches and interpret new observations in a rapidly evolving epidemiologic context.1
Physiology of the spleen
The splenic microcirculation
Five liters of blood (approximately 2 L of RBCs) circulate through the heart every minute, with 5% going to the spleen.4 A RBC therefore crosses the spleen, on average, every 20 minutes. From the splenic artery, RBCs flow to the medium-sized central artery (Figures 1–2) then engage into parallel, fast, or slow microcirculations.4 In the fast microcirculation, RBCs transit from the perifollicular zone to the venous sinus lumen through direct by-passes and are exposed to mechanical challenges not markedly different from that operating in capillaries (Figures 1–2). RBC velocity is approximately 20× greater in the fast than in the slow microcirculation.4 In the slow open microcirculation, RBCs navigate in the cords of the red pulp before returning to the vascular beds by squeezing between endothelial cells in the wall of sinuses.4-6 Crossing splenic interendothelial slits is the most stringent challenge on RBC deformability in the body7 and can result in the retention of less deformable RBCs or in removal of intraerythrocytic bodies (ie, pitting).4 Unlike the channel-shaped capillaries, the labyrinthine microcirculatory beds in the cords are devoid of endothelium, hence the “open” microcirculation. This slow, open microcirculation accounts for 10%-20% of spleen RBC flow.4,6 Quality control of RBC deformability thus occurs, on average, every 100-200 minutes, consistent with the 60-minute half-life clearance of stiff-heated RBCs observed in healthy subjects.8 Other putative signals for retention of RBCs in the slow circulation of the spleen include clustered band 3, against which autoantibodies have been found, or flipped phosphatidylserine.9,10
The splenon the functional unit of splenic RBC filtration. Blood flow is from top right to bottom left and may follow 2 parallel paths. The fast and closed circulation flows from splenic artery to central arterioles and their branches, then through the perifollicular zone (PFZ), PFZ-to-sinus by-passes, sinus lumens, postsinusal veinules that join into the splenic vein, and accounts for 80%-90% of the splenic blood flow. The slow open circulation has at least 2 specificities: (i) a microcirculatory structure devoid of endothelial cells where cord macrophages, and reticular cells survey the slowly migrating blood cells and (ii) narrow and short interendothelial slits in the sinus wall that RBC must cross to get back to the general circulation. The splenon displays analogies and differences with the nephron (see text). Schematically, the filtering function of the spleen can be divided in 3 successive steps. The “prefiltration step” (1) corresponds to the close contacts between RBCs and macrophages in the cords. RBC retention during this step is putatively triggered by ligand-receptor interactions, including direct recognition of RBC surface alterations and opsonization. The “filtration” step (2) corresponds to the crossing of interendothelial slits. RBC retention here is triggered by mechanical alterations. Undeformable bodies can also be extracted from RBCs in a process called pitting. The “postfiltration” step (3) corresponds to the modifications and processing of retained RBCs. Both the prefiltration and postfiltration steps potentially result in the phagocytosis of abnormal, decorated, or opsonized RBCs. Phagocytosis of parasitized RBCs is an initial step of antigen presentation to immune cells, thereby connecting filtration to the antigen-specific response.
The splenon the functional unit of splenic RBC filtration. Blood flow is from top right to bottom left and may follow 2 parallel paths. The fast and closed circulation flows from splenic artery to central arterioles and their branches, then through the perifollicular zone (PFZ), PFZ-to-sinus by-passes, sinus lumens, postsinusal veinules that join into the splenic vein, and accounts for 80%-90% of the splenic blood flow. The slow open circulation has at least 2 specificities: (i) a microcirculatory structure devoid of endothelial cells where cord macrophages, and reticular cells survey the slowly migrating blood cells and (ii) narrow and short interendothelial slits in the sinus wall that RBC must cross to get back to the general circulation. The splenon displays analogies and differences with the nephron (see text). Schematically, the filtering function of the spleen can be divided in 3 successive steps. The “prefiltration step” (1) corresponds to the close contacts between RBCs and macrophages in the cords. RBC retention during this step is putatively triggered by ligand-receptor interactions, including direct recognition of RBC surface alterations and opsonization. The “filtration” step (2) corresponds to the crossing of interendothelial slits. RBC retention here is triggered by mechanical alterations. Undeformable bodies can also be extracted from RBCs in a process called pitting. The “postfiltration” step (3) corresponds to the modifications and processing of retained RBCs. Both the prefiltration and postfiltration steps potentially result in the phagocytosis of abnormal, decorated, or opsonized RBCs. Phagocytosis of parasitized RBCs is an initial step of antigen presentation to immune cells, thereby connecting filtration to the antigen-specific response.
Structure-function correspondences in the slow, open microcirculation. (A) On human spleen sections, the medium-sized central artery (CA) is surrounded by white pulp (here, a lymphoid nodule; LN) made of densely packed white cells, between which no RBCs circulate. In the perifollicular zone (PFZ) that surrounds the white pulp, RBCs are visible within flat, concentric microcirculatory spaces. In the red pulp (RP), typical sinuses (S) are observed. RBCs engaged in the fast, closed circulation transit through the PFZ, whereas RBCs in the open microcirculation navigate slowly in the RP cords before returning to the blood vascular bed by crossing the wall of sinuses. Because direct by-passes exist between the PFZ and sinuses, sinus lumens (sl)—though located in the RP—collect both the fast (closed) and the slow (open) microcirculations (Giemsa-stained human spleen section ×400). (B) Macrophages account for almost half the volume of the cords (co), although approximately 10% of them can be found in the sinus lumen (sl, immunohistochemistry of an isolated-perfused human spleen section using an anti-CD68 primary antibody revealed with a peroxidase secondary antibody, ×400). Because the RP accounts for 75% of spleen volume, an average 150-g human spleen contains approximately 50 g (or 50 mL) of macrophages (ie, approximately 100 times greater than the average 0.5-mL volume of monocytes circulating in the vascular beds). (Ci) The visualization of sinus walls on histologic sections is facilitated by PAS (phosphatase acid shift) staining that highlights the peculiar basal fibers of sinuses, providing a clear separation between cords (co) and sinus lumens (sl) (isolated-perfused human spleen ×1000). The sinus wall is made of elongated endothelial cells surrounded by helical basal fibers. (Cii) In a patient treated with artemisinin derivatives, if the parasite load is high, parasite remnants either pitted from their host RBCs or retained with their host RBC delineate the abluminal side of the sinus wall (D1, PAS-stained post mortem spleen sample, ×400). (Di) On transmission electron microscopy (TEM), the periodic disposition and homogenous aspect of basal fibers (pseudocolored purple), as well as elongated shape of sinus endothelial cells (pseudocolored blue), allows an accurate orientation (isolated-perfused human spleen, ×2000). (Dii) When pitted from an RBC squeezing through an inter endothelial slit in a sinus wall, P falciparum remnants are deposited on the abluminal side of the sinus (PAS-stained section of an isolated-perfused human spleen challenged with artesunate-exposed P falciparum–infected RBCs, ×1000, inset ×2000). These observations confirm that pitting occurs exclusively or very predominantly, whereas RBCs cross the sinus wall and illustrates the unidirectional aspect of sinus wall crossing by RBCs—from cords to sinus lumen.
Structure-function correspondences in the slow, open microcirculation. (A) On human spleen sections, the medium-sized central artery (CA) is surrounded by white pulp (here, a lymphoid nodule; LN) made of densely packed white cells, between which no RBCs circulate. In the perifollicular zone (PFZ) that surrounds the white pulp, RBCs are visible within flat, concentric microcirculatory spaces. In the red pulp (RP), typical sinuses (S) are observed. RBCs engaged in the fast, closed circulation transit through the PFZ, whereas RBCs in the open microcirculation navigate slowly in the RP cords before returning to the blood vascular bed by crossing the wall of sinuses. Because direct by-passes exist between the PFZ and sinuses, sinus lumens (sl)—though located in the RP—collect both the fast (closed) and the slow (open) microcirculations (Giemsa-stained human spleen section ×400). (B) Macrophages account for almost half the volume of the cords (co), although approximately 10% of them can be found in the sinus lumen (sl, immunohistochemistry of an isolated-perfused human spleen section using an anti-CD68 primary antibody revealed with a peroxidase secondary antibody, ×400). Because the RP accounts for 75% of spleen volume, an average 150-g human spleen contains approximately 50 g (or 50 mL) of macrophages (ie, approximately 100 times greater than the average 0.5-mL volume of monocytes circulating in the vascular beds). (Ci) The visualization of sinus walls on histologic sections is facilitated by PAS (phosphatase acid shift) staining that highlights the peculiar basal fibers of sinuses, providing a clear separation between cords (co) and sinus lumens (sl) (isolated-perfused human spleen ×1000). The sinus wall is made of elongated endothelial cells surrounded by helical basal fibers. (Cii) In a patient treated with artemisinin derivatives, if the parasite load is high, parasite remnants either pitted from their host RBCs or retained with their host RBC delineate the abluminal side of the sinus wall (D1, PAS-stained post mortem spleen sample, ×400). (Di) On transmission electron microscopy (TEM), the periodic disposition and homogenous aspect of basal fibers (pseudocolored purple), as well as elongated shape of sinus endothelial cells (pseudocolored blue), allows an accurate orientation (isolated-perfused human spleen, ×2000). (Dii) When pitted from an RBC squeezing through an inter endothelial slit in a sinus wall, P falciparum remnants are deposited on the abluminal side of the sinus (PAS-stained section of an isolated-perfused human spleen challenged with artesunate-exposed P falciparum–infected RBCs, ×1000, inset ×2000). These observations confirm that pitting occurs exclusively or very predominantly, whereas RBCs cross the sinus wall and illustrates the unidirectional aspect of sinus wall crossing by RBCs—from cords to sinus lumen.
A structure-function paradigm for RBC filtration by the spleen
Here, we name the structure-function paradigm for the splenic microcirculation and RBC-filtering function, which has been progressively elaborated4,11 as the “splenon” by analogy with the nephron. We focus on its RBC-filtering function and propose successive functional steps: “prefiltration,” “filtration,” and “postfiltration” (Figure 1). The nephron and the splenon both filter materials, but the outcome differs. Although the nephron excludes the filtered elements in the urine, the splenon retains them in the red pulp. This imposes the destruction and recycling of retained RBCs, a process likely mediated predominantly by macrophages. Macrophages account for approximately half the volume of the cords4 ; their abundance facilitates direct RBC–macrophage interactions and the processing of at least 20 mL of RBCs per day (Figure 2B) under physiological conditions. The peculiarities of the milieu in the cords possibly play a role in RBC modifications that follow retention, ultimately resulting in phagocytosis.4 The ability of the spleen to innately filter out altered RBCs, either through ligand-receptor interactions or by sensing their abnormal mechanical properties, is likely a key process during infection by P falciparum. Splenic clearance of P falciparum–altered RBCs can, indeed, start very early during infection and may help raise an antigen-specific response through phagocytosis of infected RBCs (iRBCs) and parasite debris by antigen-presenting cells. In addition, the spleen also senses and, possibly, processes noninfected RBCs altered by parasite-derived products.
P falciparum–induced changes to the RBCs
Parasite stages and cytoadherence
The pathogenesis of human P falciparum infection is a complex interplay of parasite-induced RBC alterations2 and microcirculatory abnormalities,12 accompanied by local and systemic immune reactions, resulting in multiple clinical forms of variable severity.13 RBC infected with early parasite stages (rings) display mild modifications of adhesion and/or deformability properties and may circulate, whereas late parasite stages, called trophozoites and schizonts (mature forms), have substantial alterations of adhesion/deformability that favor their sequestration in small vessels, thus preventing their circulation in the peripheral blood.2 Sequestration of mature forms is induced by their adherence to endothelial cells, blood cells, platelets, and uninfected RBCs (uRBCs). These interactions are mediated by multiple host receptors recognized by parasite adhesins.2
PfEMP1, sequestration and acquired protection
The major parasite adhesin is the variant P falciparum Erythrocyte Membrane Protein 1 (PfEMP1) encoded by the var multigene family.2,14-16 The expression of PfEMP1 on mature forms is concentrated at electron-dense knobs on the iRBC membrane.2 At the end of the cycle, schizont rupture releases antigens and components that elicit a strong systemic inflammatory response, which indirectly contributes to pathogenesis by stimulating the expression of inducible endothelial receptors, thereby promoting further cytoadherence.17 When sequestered, mature forms do not circulate and escape retention and destruction by the spleen.18 Indeed, mature forms are undeformable18 and unable to cross interendothelial slits of the spleen. Interestingly, mature forms of P vivax are more deformable than normal RBCs and are observed in the peripheral circulation.19 Sequestration of mature forms of P falciparum (ie, escape from the spleen) indirectly favors parasite multiplication and explains the initial Log-linear increase of parasite loads in nonimmune subjects with P falciparum malaria.20 In patients with cerebral malaria sequestration of mature forms in small vessels,21,22 and total body parasite biomass are greater than in patients with uncomplicated attacks,23 or other severe attacks.24,25 P falciparum infection is a highly dynamic process, both in space and time. In naïve subjects with synchronous parasite multiplication, parasitemia increases or decreases by several orders of magnitude within a few hours as a consequence of invasion of new RBCs or sequestration of mature forms, respectively.26 Importantly, PfEMP1 is a highly immunogenic molecule eliciting a strong antibody response that contributes to immune protection. With repeated exposures, children in endemic areas progressively acquire antibodies against frequent PfEMP1 variants, a process thought to contribute to protection against severe disease.27
Deformability of infected and uninfected RBCs
The outcome of P falciparum malaria is not solely determined by the sequestration ability of mature forms, and there is a wide spectrum of manifestations of severe malaria, in addition to cerebral malaria.13 In particular, severe malarial anemia, the earliest, most frequent severe manifestation of P falciparum infection in hyperendemic areas,28 results from massive RBC loss and/or impaired erythropoiesis.29 Rupture of iRBCs upon merozoite release is, in fact, a smaller contributor to RBC loss than the destruction of uRBCs.30 Indeed, rings and uRBC are modified by P falciparum.2,3,18 Interactions between parasite proteins and host proteins in the cortical cytoskeleton of RBCs modify the membrane, such that iRBC deformability is mildly and markedly reduced in rings and mature forms, respectively.18,31-33 The moderate, but significant, decrease of ring deformability has been deemed to trigger their retention in the spleen,18 although this hypothesis did not gain support because of its apparent inconsistency with the observation of rings in the peripheral circulation. However, recent studies in isolated-perfused human spleens did document spleen retention of a substantial proportion of rings,6 indicating that the reduced deformability of rings could, indeed, trigger their clearance. Because this retention process corresponds to the clearance of circulating parasites, it likely reduces the speed of parasite-load increase and therefore the pace at which infection proceeds.34
The deformability of uRBCs present in parasitized blood is also mildly altered in vitro32 and in vivo.33,35 A proportion of uRBCs from parasite cultures are “decorated” with parasite molecules released during invasion.3,36,37 Such less deformable or decorated uRBCs are susceptible to splenic retention, phagocytosis, or complement-mediated lysis in the presence of specific antibodies.3,37 These processes likely exacerbate RBC loss in malaria patients.
In summary, multiple times during their lifespan, both rings and uRBCs interact with capillaries in systemic and pulmonary circulations, and with the “open” microcirculation of the spleen. This may influence not only blood flow and RBC survival,35 but also the kinetics of infection and therefore may impact on the clinical pattern of malaria and its severity.6,34,35
Not least, the mechanical sensing by the spleen of RBCs harboring the sexual stages of the parasite (ie, immature and mature gametocytes) may influence transmission. Immature gametocytes have been observed in the peripheral blood of splenectomized, but not of spleen-intact, P falciparum–infected patients.38,39 By contrast, mature gametocytes are frequently observed in the peripheral blood of spleen-intact patients. The spleen may therefore enhance the cytoadherence of immature gametocytes or retain mechanically those that do not cytoadhere. Mature gametocytes likely become less cytoadherent or more deformable (or both) than immature gametocytes. Both features are, indeed, necessary for the sustained circulation of mature gametocytes in the periphery, an essential prerequisite for parasite transmission from the human host to the Anopheles vector.
The spleen in P falciparum malaria
Clinical and pathological features
Splenomegaly is the main clinical marker of endemicity in P falciparum transmission areas,40 although palpable splenomegaly is frequently absent during acute infection in nonimmune subjects.30,41 At the time of death, adult patients with severe malaria have larger spleens than patients who died from sepsis.42 Very rarely, malarial splenomegaly leads to pathological splenic rupture.43 Macroscopically, the spleen is brown, a color change caused by pigment deposition, the hallmark of malaria on microscopic examination.44 Pigment is predominantly found in macrophages and in intact mature forms.44-46 Increased spleen weight is associated with expansion of both the red and the white pulp.42 The red pulp is congested with RBCs,47 and the relative number of macrophages is increased.42 The architecture of the white pulp is markedly disorganized, with dissolution of the marginal zone and relative loss of B cells.42 At the acute phase of fatal infection, uRBC, knob-positive and -negative iRBCs accumulate in the spleen more intensely than in the liver.45 Typical cytoadherence of mature forms occurs on the luminal side of the sinus endothelium.45 Electronic microscopy has shown iRBCs containing either morphologically normal parasites or parasite remnants squeezing through interendothelial slits and, possibly, being retained in that position (Figure 1).42,45 Whether mature forms are phagocytosed predominantly as intact iRBCs or after iRBC lysis is not known. Phagocytosis of uRBCs is a frequent finding.46
Limitations of classical observational approaches
Pathology studies can only provide static snapshot analyses at terminal stages of severe disease, furthermore in patients having received antimalarial therapy. Therefore, pathological signs observed at the time of death reflect retention and processing of RBCs as well as white blood cell multiplication or migration (or both) over several days. In addition, these observations are superimposed in the geometrically complex space of the red pulp. Except for the accumulation of iRBCs or parasite remnants along the abluminal side of the sinus walls, which likely corresponds to mechanical retention6 or pitting5 (Figure 2D), mechanistic interpretation of findings is difficult.
Experimental studies in malaria patients
Four experimental studies in Thai adults, which determined the clearance kinetics of labeled RBC at different stages of P falciparum attacks,8,48-50 highlight the role of the spleen in RBC survival during P falciparum infection. In uncomplicated malaria patients with splenomegaly who had not yet received antimalarial therapy, the clearance of heat-stiffened autologous RBCs was enhanced. In patients without splenomegaly, enhanced clearance was only observed after drug therapy.8 There was no major splenic dysfunction in these patients, and the accelerated clearance of stiff RBCs persisted long after parasite clearance.8 Just after parasite clearance,50 there was an accelerated antibody- and complement-independent clearance of labeled autologous or donor RBCs. Clearance of immunoglobulin G (IgG)–sensitized RBCs was markedly accelerated as well, and this persisted for several weeks.48 In patients with severe malaria, destruction of labeled autologous RBCs was also markedly accelerated.49 In summary, the clearance of mechanically altered-, surface-altered– and normal RBCs is accelerated in P falciparum–infected adults, a process influenced by antimalarial therapy and splenomegaly. The underpinning abrupt modifications of circulatory pathways, macrophage number, or activation status were not determined. Because specific labeling of iRBCs was not feasible, these impressive experimental studies allow only indirect inference on the mechanisms of iRBCs clearance by the spleen.
Acute malaria attacks in splenectomized subjects: general
A powerful approach to grasp the physiologic or pathogenic role of an organ is to study what happens when it is absent. The analysis of published data (summarized in Table 1) shows that among 94 splenectomized patients with P falciparum infection (corresponding to 17 articles38,39,51-65 ), 3 were experiencing their first malaria attack (“naïve”) and 22 had few previous attacks or undocumented history (and “possibly immune”), and 69 had been living in an endemic area for more than 8 years before splenectomy (“immune”),63-65 and 3 experienced a switch from chronic carriage to symptomatic attack within 3-8 weeks after splenectomy.39,62 Splenectomy also affects the clearance of iRBCs after antimalarial treatment.58-61 Data and interpretation on each of these different situations are analyzed separately in Table 1 and below (Figure 3).
Clinical and parasitological features of P falciparum malaria in splenectomized subjects
| Severity and fatality . | Incidence of “malaria” and febrile symptoms . | Parasite stage distribution on blood smears . | Ultrastructural and molecular studies of iRBCs . |
|---|---|---|---|
| Patients naïve or possibly immune before splenectomy: case reports or series of cases70,83-93 (12 articles 25 patients) | |||
| Overall, 48% of patients had severe malaria and 28% died; 7 of 25 patients had cerebral malaria. | Not relevant (only patients with symptoms were reported) | Mean and median parasitemia = 17% and 5% (13 patients); mature forms accounted for > 3% (often > 20%) of asexual forms in 6 of 10 patients from whom the information was available | Infected erythrocytes allowed to mature in vitro had knobs (electronic microscopy) and were able to cytoadhere in a static assay93 ; no knobs observed on iRBCs in an autopsy study performed after 13 days of antimalarial therapy86 |
| In unsplenectomized travelers, the severity rate is 5%-10% and death rate 0.5%-5%. | In unsplenectomized patients with uncomplicated, severe, and fatal attacks, the mean proportion of mature forms is 0.03%, 0.3%, and 3% of asexual forms, respectively53 | ||
| Immune patients: cohort studies95-97 for 3 articles (69 patients and 83 controls) | |||
| Five deaths in 69 patients followed for 1-10 years | Fever and parasitemia are significantly more frequent in splenectomized subjects95 than in age-, sex-, and exposure-matched controls | Late stages account for > 1/3 of asexual stages in 20.6% of positive smears vs. 0% in controls95 | |
| Acute attack after splenectomy in a chronic carrier: case reports and short series of cases70,94 for 2 articles (3 patients) | |||
| One of 3 patients had severe malaria; none died | Not relevant (only patients with symptoms were reported) | Parasitemia was 0.1%, 2%, and 24%; mature forms accounted for 80% of asexual forms in 1 patient; immature gametocytes were present in 1 patient | Transcription of var, rif A, and stevor gene families was absent just after sampling but reappeared after adaptation of the isolate in culture94 ; same pattern in a static cytoadherence test in vitro94 |
| Severity and fatality . | Incidence of “malaria” and febrile symptoms . | Parasite stage distribution on blood smears . | Ultrastructural and molecular studies of iRBCs . |
|---|---|---|---|
| Patients naïve or possibly immune before splenectomy: case reports or series of cases70,83-93 (12 articles 25 patients) | |||
| Overall, 48% of patients had severe malaria and 28% died; 7 of 25 patients had cerebral malaria. | Not relevant (only patients with symptoms were reported) | Mean and median parasitemia = 17% and 5% (13 patients); mature forms accounted for > 3% (often > 20%) of asexual forms in 6 of 10 patients from whom the information was available | Infected erythrocytes allowed to mature in vitro had knobs (electronic microscopy) and were able to cytoadhere in a static assay93 ; no knobs observed on iRBCs in an autopsy study performed after 13 days of antimalarial therapy86 |
| In unsplenectomized travelers, the severity rate is 5%-10% and death rate 0.5%-5%. | In unsplenectomized patients with uncomplicated, severe, and fatal attacks, the mean proportion of mature forms is 0.03%, 0.3%, and 3% of asexual forms, respectively53 | ||
| Immune patients: cohort studies95-97 for 3 articles (69 patients and 83 controls) | |||
| Five deaths in 69 patients followed for 1-10 years | Fever and parasitemia are significantly more frequent in splenectomized subjects95 than in age-, sex-, and exposure-matched controls | Late stages account for > 1/3 of asexual stages in 20.6% of positive smears vs. 0% in controls95 | |
| Acute attack after splenectomy in a chronic carrier: case reports and short series of cases70,94 for 2 articles (3 patients) | |||
| One of 3 patients had severe malaria; none died | Not relevant (only patients with symptoms were reported) | Parasitemia was 0.1%, 2%, and 24%; mature forms accounted for 80% of asexual forms in 1 patient; immature gametocytes were present in 1 patient | Transcription of var, rif A, and stevor gene families was absent just after sampling but reappeared after adaptation of the isolate in culture94 ; same pattern in a static cytoadherence test in vitro94 |
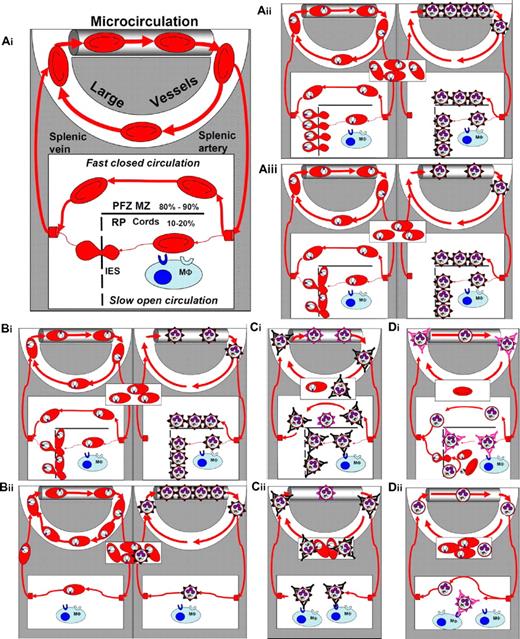
Interactions of P falciparum–infected RBCs with the microcirculation, and with the spleen during acute and chronic infection in patients with normal or impaired splenic function. (Ai) Simplified frame from Figure 1. For simplicity, all nonsplenic microcirculatory structures—either systemic or pulmonary—have been considered homogenous and are presented as a single microvascular channel at the top of the panel (PFZ MZ: perifollicular or marginal zones; RP: red pulp; interendothelial slits: interendothelial slits). Of note, large vessels correspond to the only compartment that can routinely be explored for the presence of infected RBCs (thin and thick smears, or PCR). Forms observed in the rectangle in the middle of the panel symbolize what is usually observed on patient smears in the corresponding situation. (Aii) Situation in a naïve patient (see text for definition) with a normal spleen function. Interactions are shown separately for the young ring forms (rings, left panel) and the mature forms (right panel). (Aiii) Same situation as above, but integrating the retention of a proportion of rings upstream from interendothelial slits, as recently observed in an ex vivo human spleen model.6 (Bi-ii) Comparative modeling of situations during the first acute infection in unsplenectomized (Bi top panel identical to panel Aiii) and splenectomized patients (Bii lower panel). (Ci-ii) Comparison of acute infection in immune unsplenectomized (panel Bi identical to panel Aiii) or splenectomized patients still exposed to P falciparum transmission after splenectomy (Bii). (Di-ii) Putative mechanisms of acute malaria attacks occurring a few weeks after splenectomy in chronic carriers no longer exposed to P falciparum transmission.
Interactions of P falciparum–infected RBCs with the microcirculation, and with the spleen during acute and chronic infection in patients with normal or impaired splenic function. (Ai) Simplified frame from Figure 1. For simplicity, all nonsplenic microcirculatory structures—either systemic or pulmonary—have been considered homogenous and are presented as a single microvascular channel at the top of the panel (PFZ MZ: perifollicular or marginal zones; RP: red pulp; interendothelial slits: interendothelial slits). Of note, large vessels correspond to the only compartment that can routinely be explored for the presence of infected RBCs (thin and thick smears, or PCR). Forms observed in the rectangle in the middle of the panel symbolize what is usually observed on patient smears in the corresponding situation. (Aii) Situation in a naïve patient (see text for definition) with a normal spleen function. Interactions are shown separately for the young ring forms (rings, left panel) and the mature forms (right panel). (Aiii) Same situation as above, but integrating the retention of a proportion of rings upstream from interendothelial slits, as recently observed in an ex vivo human spleen model.6 (Bi-ii) Comparative modeling of situations during the first acute infection in unsplenectomized (Bi top panel identical to panel Aiii) and splenectomized patients (Bii lower panel). (Ci-ii) Comparison of acute infection in immune unsplenectomized (panel Bi identical to panel Aiii) or splenectomized patients still exposed to P falciparum transmission after splenectomy (Bii). (Di-ii) Putative mechanisms of acute malaria attacks occurring a few weeks after splenectomy in chronic carriers no longer exposed to P falciparum transmission.
Acute malaria attacks in splenectomized “naïve” and “possibly immune” subjects
During P falciparum primary infections in splenectomized patients, severity and death are more frequent, parasitemia is higher, and mature forms circulate more frequently than in nonsplenectomized patients. We found 25 cases in the literature (Table 1). These data suggest that the spleen plays a major protective role in naïve patients. Cerebral malaria is frequent in this context (7 patients of 25) (Table 1), and the expression of knobs and surface adhesins on iRBCs is not altered in splenectomized patients.61 Thus, the higher frequency of circulating mature forms in splenectomized patients (Table 1) probably reflects the lack of splenic clearance, rather than defective sequestration. In the absence of antibody to iRBC surface antigens in naïve patients, clearance of mature forms in the spleen likely involves innate ligand-receptor interactions with macrophages66 or mechanical retention upstream from interendothelial slits (Figure 3Aii-Aiii).18 The presence of higher parasitemias in splenectomized patients also supports the hypothesis that the spleen innately retains a proportion of rings,34 reducing the number of mature forms that cytoadhere in small vessels a few hours later (Figure 3Aiii). In summary, a lack of splenic retention of rings, such as mature forms (or both), likely explains why parasitemia is higher, and infection more severe, in splenectomized than in spleen-intact naïve patients.
Acute malaria attacks in splenectomized immune subjects
In areas endemic for P falciparum, fever and parasitemia are significantly more frequent in splenectomized subjects than in spleen-intact relatives (Table 1).63,64 Severity and fatality of P falciparum infection is possibly increased, but not to a large extent (Table 1). These data have been interpreted as follows.63 In immune patients with a spleen as well as in splenectomized patients (Figure 3Ci-Cii), antibodies to surface adhesins of mature forms impede their sequestration in the microcirculation, thereby preventing severe complications. In spleen-intact patients, the mature forms, unable to cytoadhere, are cleared by the spleen. Moreover, the acquired antibody-dependent protection27,67 combines with “antitoxic” immunity to reduce inflammation-dependent symptoms and complications. Retention in the spleen may be favored by opsonization in the macrophage-rich cords or mechanical trapping upstream from interendothelial slits, the latter potentially enhancing the former. In splenectomized immune patients, however (Figure 3Cii), mature forms are less efficiently cleared either because the microcirculation in other organs cannot mechanically retain mature forms, or because opsonization is less efficient in sinusoids of the liver, or other macrophage-rich organs than in the splenic cords. Taken together, these observations show that the spleen plays a specific role in the control of P falciparum parasite loads, even in the presence of pre-existing acquired protection. This spleen-specific role does not account for the whole protection, however, as severity is not the rule. Other organs take over for the control of P falciparum loads, but act less efficiently than does the spleen.
Acute malaria attacks in chronic carriers undergoing splenectomy
Acute malaria attacks occurring a few weeks after splenectomy in chronic carriers no longer exposed to P falciparum transmission have been described.39,62 In the single patient in whom this has been explored to date, there was no expression of genes for surface adhesins, and cytoadherence of iRBCs during the first cycle after sample collection, but both were progressively restored in culture.39 This was interpreted as reflecting the positive selection for rare variants in immune patients with a normal spleen function (Figure 3Ci), which if P falciparum carriage is maintained long enough without reinfection ultimately leads to the selection of adhesin-less variants (Figure 3Di) that possibly escape clearance and destruction by the spleen.39 How can such adhesin-less mature forms multiply without being cleared by the spleen? This may result either from their mechanical retention in the microvasculature or from some low-grade intrasplenic development with reinvasion in the RBC-rich cords, giving rise to a new, low-grade wave of parasites (Figure 3Di). This hypothesis is consistent with important deposits of malaria pigment in the spleen of the studied patient.39 The existence of such an intrasplenic cryptic cycle fits well with the major features of hyper-reactive malaria splenomegaly namely, massive splenomegaly, absence of fever, absent or rare parasites on blood smears, high levels of specific antibodies, very high levels of total immunoglobulin M, and positive outcome after sustained antimalarial therapy.68 When the spleen is removed in such patients, mechanical retention, the only powerful mechanism able to clear adhesin-less variants, disappears, giving rise to a rapid increase of parasite loads and an acute clinical attack (Figure 3Dii).
Clearance of iRBCs (parasite clearance) in drug-treated splenectomized patients
Regardless of the antimalarial agent used (eg, artesunate, or quinine, sometimes associated with tetracycline or mefloquine), parasite clearance time is markedly prolonged in splenectomized patients, taking several weeks, instead of a very few days, in patients with a spleen. In a splenectomized patient,54 parasitemia only mildly decreased from 63% to 30% after 13 days of treatment, confirming that the spleen is the essential player for rapid posttherapeutic parasite clearance.69 Studies in patients with a spleen70 have indicated that pitting of parasite remnants from the RBCs is the major determinant of parasite clearance time, especially when artemisinin derivatives are administered. These observations also confirm that pitting is a spleen-specific mechanism. Interestingly, artemisinin-induced parasite remnants are morphologically reminiscent of Howell-Jolly bodies (ie, erythroblast nuclear remnants observed on Giemsa-stained smears in a proportion of RBCs from splenectomized patients). Pitting of parasite remnants may thus result from the subversion of a physiological phenomenon (ie, removal of Howell-Jolly bodies from circulating RBCs). Rising parasite resistance to artemisinin derivatives in Southeast Asia may be partly explained by a slower transformation of ring-form parasites into undeformable parasite remnants resembling particulate bodies, thereby delaying pitting. This parasite-encoded trait71 would initially manifest itself essentially by a delayed parasite clearance time.72
Influence of the spleen on severe malaria, including anemia
Potential mechanisms of anemia in malaria
The pathogenesis of anemia in patients with P falciparum parasitemia is multifactorial. However, the efficacy of continuous or intermittent administration of antimalarial agents at preventing severe malarial anemia in African children73 has indicated that P falciparum infection is a major, direct contributor to this complication. In naïve subjects with acute P falciparum infections and without comorbidities, RBC loss is an important mechanism of anemia.30 Although often present in acute infection,74 dyserythropoiesis is likely a minor contributor to acute malarial anemia, as complete abrogation of erythropoiesis alone only results in a 1% decrease of RBC biomass per day.75 Conversely, in patients with prolonged low-grade P falciparum carriage, inhibition of erythropoiesis likely plays a predominant role.34 At the chronic end of the continuum, hypersplenism and antibody-mediated hemolysis are considered the predominant mechanisms of anemia in patients with hyper-reactive malarial splenomegaly.68 Severe malarial anemia is therefore more a syndrome than a disease, reflecting diverse pathogenic processes. Acute anemia in naïve patients likely results from fewer mechanisms, allowing a simpler analysis of pathogenesis.30 At least in some settings, severe malarial anemia has a predominantly acute evolution,28,41,76 further pointing to RBC loss as an important mechanism.
Malarial anemia and retention of RBCs in the spleen
Recently, a new paradigm was proposed,6 whereby the extent of RBC retention in the spleen, a mechanism of RBC loss, determines not only hemoglobin concentration and spleen size, but also affects parasite load. Indeed, because the retention of rings in the spleen corresponds to the clearance of circulating parasites, it likely reduces the speed of parasite load increase and therefore the pace at which infection proceeds. This connection between innate retention of rings, on the one hand, and innate retention of uRBCs, on the other, is perfectly in line with the observations that, in naïve or poorly immune subjects, splenomegaly correlates with a decreased hematocrit or hemoglobin level,8,30,41 and that duration of fever (from onset to diagnostic) is longer when anemia is present41,77
Confronting alternative hypotheses
It had also been proposed that receptors on cerebral endothelial cells—necessary for the sequestration of mature forms leading to cerebral malaria—are lacking in infants and appear later during childhood.28 Because infants lacking cerebral endothelial receptors would be innately protected from cerebral malaria, P falciparum infections would not be interrupted by coma or death, leaving more time for anemia and splenomegaly to develop. Conversely, older children would become comatose, be treated, or die before anemia could develop. In such a scenario, the higher frequency of anemia and splenomegaly in infants would mainly reflect the long progression of the infection. Two observations, however, go against this model. First, splenomegaly is associated with RBC loss independently from the duration of infection.30 Second, parasitemia is lower in children with severe malarial anemia than in those with cerebral malaria,41 despite the longer duration of fever in the former.30,41,77 The hypothesis that age modulates the stringency of splenic retention of rings and uRBCs reconciles more epidemiologic observations than does the lack of a cerebral receptor in infants.34 It predicts that the risk of cerebral malaria increases and the risk of severe malarial anemia decreases as children grow older,78 strongly suggesting that the same mechanism may altogether precipitate severe malarial anemia and protect against cerebral malaria.34 Figure 4 illustrates the link between the clearance of uRBCs (a determinant of acute anemia) and that of rings (a determinant of slower evolution) and outlines the potentially pathogenic impact of variations in either RBC deformability or stringency of splenic sensing of RBCs in physiologic or disease states. Both age and hemoglobin polymorphisms may be associated with such variations (Figure 4).
Ability of RBCs to cross the spleen, dispersion of individual values, and possible variability of the splenic detection threshold in the context of P falciparum infection. (A) The speculative distribution of mature RBCs with different levels of splenic “crossability” at homeostasis. Approximately 1% of the circulating RBC population loses its ability to cross the spleen each day. When the spleen crossability of a RBC is below the splenic retention threshold, the corresponding RBC is retained in the cords of the red pulp. This retention is expected to trigger phagocytosis, followed by iron recycling into erythropoiesis. Aging of a RBC is associated with a progressive reduction of RBC deformability that influences RBC crossability.99 A link between deformability (assessed by ektacytometry) and spleen crossability has been established in thalassaemia.100 (B) 1. The elongation of ring-infected RBCs is reduced18 with a wide dispersion of individual values, as assessed using optical tweezers.32 Deformability values of rings span the splenic retention threshold, as shown by the retention of rings in an ex vivo human spleen-perfusion system.6 2. The splenic crossability of a proportion of the ring population is therefore below the splenic retention threshold. This subpopulation is retained and (at least partially) phagocytosed, therefore unable to sequester in the vasculature of other organs. 3. The subpopulation that is still able to circulate is available for maturation and subsequent cytoadherence-based sequestration. Despite their gross inability to cross the spleen, cytoadherent mature forms are protected from splenic retention by sequestration in the microcirculation. 4. The biomass of sequestered mature forms generated at each cycle thus depends on the proportion of circulating rings and determines the number of rings produced at the next reinvasion wave.5 The proportion of rings allowed to circulate through the spleen is therefore a determinant of the in vivo multiplication factor of the parasite biomass. (C) Influence of a left shift in the crossability of RBCs. Several RBC disorders/polymorphisms are associated with a reduced RBC deformability or decreased ability of RBCs to cross the spleen (1). This abnormality may induce a similar shift in the deformability of ring-infected RBCs. The subpopulation of rings retained is therefore greater, and the subpopulation allowed to circulate until maturation and sequestration is smaller, giving rise to a smaller sequestered biomass of mature forms. The number of rings generated at the next reinvasion cycle is also smaller, leading to a reduced in vivo multiplication factor. This process may account for part of the protection from severe malaria induced by the HbAS trait (see text). (D) In an anemic condition, when a greater number of normal RBCs are retained in the spleen, the spleen clearance zone will be shifted to the right. If the splenicretention threshold is higher (1) (ie, the spleen retains RBCs with a higher crossability), the subpopulation of rings retained is greater (2), and the subpopulation allowed to circulate until maturation and sequestration is smaller (3), giving rise to a smaller sequestered biomass of mature forms. The number of rings generated at the next reinvasion cycle is also smaller, leading to a reduced in vivo multiplication factor. A more stringent spleen retention threshold also induces the retention of a greater proportion of normal RBCs, thereby favoring the occurrence of anemia. (E) Uninfected RBCs in the blood of patients or in culture usually have a reduced deformability (1). The population distribution curve of uninfected RBCs is therefore shifted to the left, a phenomenon predicted to increase the proportion of uninfected RBCs retained in the spleen, and thereby RBC loss and subacute/acute anemia. The existence of this third subpopulation of RBCs explains how anemia and a decrease of the deformability of circulating RBCs can be associated.6,34 Whether this process affecting uninfected RBCs is unimodal or multimodal is not known.
Ability of RBCs to cross the spleen, dispersion of individual values, and possible variability of the splenic detection threshold in the context of P falciparum infection. (A) The speculative distribution of mature RBCs with different levels of splenic “crossability” at homeostasis. Approximately 1% of the circulating RBC population loses its ability to cross the spleen each day. When the spleen crossability of a RBC is below the splenic retention threshold, the corresponding RBC is retained in the cords of the red pulp. This retention is expected to trigger phagocytosis, followed by iron recycling into erythropoiesis. Aging of a RBC is associated with a progressive reduction of RBC deformability that influences RBC crossability.99 A link between deformability (assessed by ektacytometry) and spleen crossability has been established in thalassaemia.100 (B) 1. The elongation of ring-infected RBCs is reduced18 with a wide dispersion of individual values, as assessed using optical tweezers.32 Deformability values of rings span the splenic retention threshold, as shown by the retention of rings in an ex vivo human spleen-perfusion system.6 2. The splenic crossability of a proportion of the ring population is therefore below the splenic retention threshold. This subpopulation is retained and (at least partially) phagocytosed, therefore unable to sequester in the vasculature of other organs. 3. The subpopulation that is still able to circulate is available for maturation and subsequent cytoadherence-based sequestration. Despite their gross inability to cross the spleen, cytoadherent mature forms are protected from splenic retention by sequestration in the microcirculation. 4. The biomass of sequestered mature forms generated at each cycle thus depends on the proportion of circulating rings and determines the number of rings produced at the next reinvasion wave.5 The proportion of rings allowed to circulate through the spleen is therefore a determinant of the in vivo multiplication factor of the parasite biomass. (C) Influence of a left shift in the crossability of RBCs. Several RBC disorders/polymorphisms are associated with a reduced RBC deformability or decreased ability of RBCs to cross the spleen (1). This abnormality may induce a similar shift in the deformability of ring-infected RBCs. The subpopulation of rings retained is therefore greater, and the subpopulation allowed to circulate until maturation and sequestration is smaller, giving rise to a smaller sequestered biomass of mature forms. The number of rings generated at the next reinvasion cycle is also smaller, leading to a reduced in vivo multiplication factor. This process may account for part of the protection from severe malaria induced by the HbAS trait (see text). (D) In an anemic condition, when a greater number of normal RBCs are retained in the spleen, the spleen clearance zone will be shifted to the right. If the splenicretention threshold is higher (1) (ie, the spleen retains RBCs with a higher crossability), the subpopulation of rings retained is greater (2), and the subpopulation allowed to circulate until maturation and sequestration is smaller (3), giving rise to a smaller sequestered biomass of mature forms. The number of rings generated at the next reinvasion cycle is also smaller, leading to a reduced in vivo multiplication factor. A more stringent spleen retention threshold also induces the retention of a greater proportion of normal RBCs, thereby favoring the occurrence of anemia. (E) Uninfected RBCs in the blood of patients or in culture usually have a reduced deformability (1). The population distribution curve of uninfected RBCs is therefore shifted to the left, a phenomenon predicted to increase the proportion of uninfected RBCs retained in the spleen, and thereby RBC loss and subacute/acute anemia. The existence of this third subpopulation of RBCs explains how anemia and a decrease of the deformability of circulating RBCs can be associated.6,34 Whether this process affecting uninfected RBCs is unimodal or multimodal is not known.
P falciparum–human coevolution: the influence of the spleen on innate immunity
Age as an independent factor of malaria severity
In high transmission areas, P falciparum infection is severe only in young children, likely because older children and adults are protected by acquired immunity.27,28 Unexpectedly, in nonimmune transmigrants,79 travelers,80 and poorly immune patients in Southeast Asia81 or Vanuatu,82 P falciparum infection is less frequently severe or fatal in children than in adults. In patients with severe malaria,81 hyperparasitemia and mortality incidence increases with age, whereas severe malarial anemia is more frequent in children and less frequently fatal than cerebral malaria.13,81 Older patients may have less efficient parasite clearance mechanisms and less efficient clearance of noninfected RBCs than do younger patients.81 In summary, age of the host as a risk factor for cerebral malaria and severe malarial anemia is partially independent from the intensity of transmission,78 and thus not only reflects acquisition of immunity, but also age-dependent innate protective mechanisms, with an “inverted” profile (ie, naïve children have a smaller risk of life-threatening severe malaria than naïve adults). A critical determinant of lifetime disease risk is therefore “the ability to develop antigen-specific immunity early in life during a period when innate protective mechanisms may operate.”40
The Charibdis and Scylla analogy
Although the “inverted” age-dependent innate protection is thus beneficial to the host, could it also be beneficial to the parasite? The Greek myth of Charibdis and Scylla illustrates how a coevolution process may operate. On their way back home, Ulysses and his companions once navigated along a strait bordered on each side by 2 opposed threats. The cavernous rock, Charybdis, aspirates the whole boat with its entire crew, then destroys it or spits it out undamaged. Scylla, a snake-like monster, picks and kills unlucky members of the crew. Any move away from Charybdis exposes the group to Scylla and vice versa. This myth illustrates 2 concepts. First, in some situations, keeping away from one risk exposes oneself to another risk. Nonimmune children infected with P falciparum are, indeed, exposed to the distinct, partially opposed risks of cerebral malaria or severe malarial anemia.13,78 Second, when the margin of action is narrow, there may be a difficult choice between random death for all (ie, all could die or all could survive) and random risk of death for some people in the population (ie, some would certainly die, some would certainly survive). The parasite population, on its way to the transmission (as gametocytes) to another Anopheles, is exposed to 2 opposed threats: too fast an increase of parasite load resulting in death of the human host before transmission can take place, or too slow an increase resulting in too few transmissible people being produced. Charybdis symbolizes fast increase of parasite loads leading to cerebral malaria (ie, the risk of death of the host before transmission can take place). Scylla symbolizes a slower increase because of splenic retention of a proportion of rings, a mechanism killing some members of the parasite population, but leaving others alive. Depending on the proportion of rings cleared, transmission might be ultimately hampered or not. Acute malarial anemia may illustrate a “middle-strait” situation, where a moderate level of ring clearance prevents from cerebral malaria, yet maintains a reasonable parasite load increase.34 Case fatality of severe malarial anemia is, indeed, 2-5 times lower than that of cerebral malaria.13 Case fatality of moderate anemia is even lower. Thus, parasite and host adaptation processes may have selected (as did Ulysses) a navigation path passing closer to Scylla-severe malarial anemia (ie, loss of a few sailors/retained ring subpopulation) than to Charybdis-cerebral malaria (ie, risk of losing the entire crew/the entire parasite population dies with its host).
Optimal multiplication rates (ie, evolutionwise) of P falciparum—taking into account the intensity of the splenic clearance of rings—were probably selected in children, by far the largest susceptible host population. The hypothesis is therefore that, in children,83 more stringent sensing of RBC by splenic interendothelial slits, or reduced steady-state RBC deformability, or more efficient phagocytosis/killing of retained rings, would result in a slower parasite load increase than in adults.
Similarities between malaria and babesiosis
Does this age-dependent variation of iRBC clearance by the spleen exist in other contexts than malaria? The answer is yes. In bovine babesiosis, young animals are more resistant to clinical signs.84 In endemic areas where transmission is intense, most susceptible hosts become infected early in life and develop immunity to infection, giving rise to a fully protected adult herd, an important mechanism of enzootic stability.84 In areas of low transmission, severe babesiosis is more common, because some animals become infected while the window of innate resistance has passed. Like P falciparum, Babesia bovis, and B divergens infect RBCs and multiply faster in splenectomized hosts.84 Therefore, the mechanisms of “inverted” innate resistance in bovine babesiosis may be similar to that underlying the independent effect of age on severe P falciparum infection. As Babesia does not have pigmented multinucleated mature forms, the potential common mechanism of innate “inverted” protection, may rely in both cases on a spleen-dependent effect on rings (P falciparum) or “ring-like” Babesia-infected RBCs.
Spleen-filtering function and hemoglobin polymorphisms
Interestingly, another major innate protective factor, the sickle-cell trait, may involve the retention of a high proportion of rings in the spleen. A few hemoglobin polymorphisms protect against severe falciparum malaria, including the homozygous carriage of hemoglobin C,85 and the heterozygous carriage of hemoglobin S.86 We will here discuss the latter case. The malaria-specific protection conferred by heterozygous carriage (HbAS), is well established for clinical attacks and even stronger for cerebral malaria and severe malarial anemia.86 HbAS patients also have higher circulating immunoglobulin G antibody levels to P falciparum antigens than normal children,87,88 but in hyperendemic areas, HbAS-mediated protection exists before a significant level of clinical immunity is achieved.89,90 Reduced expression of surface adhesins on mature forms may explain protection against cerebral malaria, but does not directly explain protection against severe malarial anemia.91 The most convincing mechanisms underlying the wide HbAS antimalarial protection are therefore either the reduced ability of parasites to multiply in HbAS cells at low oxygen pressure or their premature removal from the circulation.86 The latter might be related to the retention of rings in the spleen. Indeed, when exposed to low-oxygen partial pressure, HbAS rings sickle much faster than HbAS uRBCs,92,93 a phenomenon likely inducing their retention by the spleen. Baseline reduction of HbAS-RBC deformability may also play a role.34 Recent evidence indicates that homozygous HbS carriage also impacts on clinical severity and infection.94,95 The prevalence and density of parasitemia were lower in children with sickle-cell anemia than in patients without sickle-cell anemia in Kenya, with a trend toward less severe forms.95 However, protection is not complete, and malaria is significantly associated with severe malarial anemia and death in hospitalized homozygous HbS patients.94 Somewhat paradoxically, the prevalence of parasitemia was identical or slightly greater in HbSS than in HbAS subjects.94-96 The lack of (or limited) protection afforded by HbSS, compared with HbAS, might be explained by 2 mutually counteracting processes: a stronger protection of HbSS subjects because of higher concentration of HbS, on the one hand, and a weaker protection because of progressive sickle-cell disease-induced hyposplenism on the other. Baseline anemia in children with sickle-cell anemia also likely contributes to disease severity. It would be interesting to enumerate pocked (or pitted) cells along with other markers of hyposplenism in HbSS children with or without acute malaria. The expectation is a weaker antimalarial protection in highly than in mildly hyposplenic HbSS children.
Conclusion
Knowledge on the physiologic RBC-filtering function of the spleen allows a more precise determination of its role in the pathogenesis of P falciparum malaria. The unique ability of the spleen to innately filter out RBC with altered mechanical properties—be they marked or mild—is consistent with its stronger protective role in naïve than in immune subjects and provides a parsimonious explanation for the RBC loss component of acute malarial anemia. Several features of malarial anemia (eg, relatively slow evolution, splenomegaly, and relatively low parasitemia) fit a model where both uRBCs and rings are innately retained. Further studies—requiring close interactions between clinicians, physicists, and immunologists—are now necessary to confirm or invalidate the hypothesis of a more stringent retention or processing of RBCs in the spleen of infants. In addition to mechanical retention (ie, filtration stricto sensu), several mechanisms of prefiltration or postfiltration of RBCs by the spleen may operate with varying efficiency at different patient ages (Figure 1), such as, for example, complement-dependent hemolysis.97 Pathogenic processes during P falciparum malaria are not limited to the splenic retention of more or less stiff RBCs. The main objective of this review was to extract from the complex pathogenesis of P falciparum infection a few simple paradigms, on which a more complete and complex conceptual framework could be later built. During future large, epidemiologic studies, precise capture of duration of disease, presence of splenomegaly, and quantitative parasitemia may also allow more accurate mechanistic interpretations. Hopefully, the insights on spleen physiology provided by studying malaria pathogenesis will be useful in other fields of hematology and immunology. We did not analyze how spleen-dependent innate mechanisms of protection may influence immunologic responses. However, we find it interesting to consider that the retention of iRBCs in the spleen likely impacts the kinetics, nature, and localization of antigen presentation, thereby influencing both the early cytokine response and the acquisition of antigen-specific adaptive immunity.98
Acknowledgments
We thank Sylvie Perrot and Sylvestre Biligui for their outstanding efficiency. We express the deepest gratitude to Geneviève Milon and Peter David for their essential support over the years, as well as for inspiring and still ongoing guidance toward accurate scientific thinking.
This work was supported by a grant from the Région Ile-de-France (to I.S.N. and G.D.) and a grant from “Fonds dédié pour les maladies parasitaires,” Sanofi/MRT (to P.B.). Project title: Mechanisms of Erythrocytic Infection and Anemia in Malaria PI Kasturi Haldar, Award Number 5P01HL078826-06 from the National Heart, Lung and Blood Institute.
National Institutes of Health
Authorship
Contribution: P.B. wrote the initial version of the manuscript, table, and figures and integrated the input from coauthors in the final version; and I.S., G.D., V.B., V.P., M.T., G.T., and O.M.-P. defined the general scope of the manuscript, generated parts of the text and figures, and revised successive manuscript versions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pierre A. Buffet, Institut Pasteur, Unité d'Immunologie Moléculaire des Parasites, Département de Parasitologie Mycologie, F-75015 Paris, France; e-mail: pabuffet@pasteur.fr.