In this issue of Blood, Tzeng et al report on the generation of adult, conditional SDF-1–deficient mice in which HSC quiescence and endosteal niche localization are impaired.1
The chemokine stromal cell–derived factor 1 (SDF-1; CXCL12), and its major receptor CXCR4, regulate many aspects of hematopoietic stem cells (HSCs), including their migration, survival, and development.2 Deletion of SDF-1 or CXCR4 alleles in murine embryos results in premature neonatal death due to multiple defects, including lack of bone marrow (BM) seeding by HSCs.3,4 Thus, the physiologic roles of SDF-1 in adult mice were poorly understood until the current study by Tzeng et al. This novel model allowed the authors to reduce SDF-1 transcript levels to less than 5% in the major murine hematopoietic sites, namely the BM and spleen.1
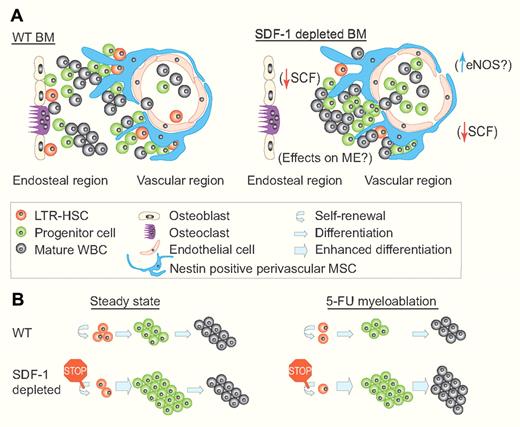
Suggested models for the regulation of hematopoiesis by SDF-1. (A) A suggested model for the regulation of hematopoiesis and BM topography by SDF-1. (Left) In WT mice, hematopoiesis is localized mainly in close proximity to the endosteal and vascular regions, where LTR-HSCs self-renew and produce more committed progenitors. These progenitors proliferate and differentiate giving rise to all kinds of mature WBCs. (Right) In SDF-1–depleted mice, hematopoiesis is restricted to the vascular region, possibly due to endosteal niche dysfunction and/or due to increased levels of phosphorylated eNOS. SCF levels are down-regulated, and the LTR-HSC pool is decreased to the benefit of the enlarged progenitor pool. How the microenvironment (ME) itself is influenced by the lack of SDF-1 is yet to be defined. (B) A suggested model for the regulation of hematopoiesis by SDF-1 in steady state and after 5-FU myeloablation. (Left) During steady state, SDF-1–depleted mice have decreased levels of LTR-HSCs probably due to reduced self-renewal. In addition, these mice possess an enlarged proliferating pool of HSPCs with no change in WBC counts. (Right) After 5-FU myeloablation, SDF-1–depleted mice exhibit enhanced recovery of both progenitors and mature WBCs, probably also at the expense of LTR-HSCs. This enhanced hematopoietic recovery provides these mice with a clear survival advantage compared with WT mice. (Professional illustration by Paulette Dennis.)
Suggested models for the regulation of hematopoiesis by SDF-1. (A) A suggested model for the regulation of hematopoiesis and BM topography by SDF-1. (Left) In WT mice, hematopoiesis is localized mainly in close proximity to the endosteal and vascular regions, where LTR-HSCs self-renew and produce more committed progenitors. These progenitors proliferate and differentiate giving rise to all kinds of mature WBCs. (Right) In SDF-1–depleted mice, hematopoiesis is restricted to the vascular region, possibly due to endosteal niche dysfunction and/or due to increased levels of phosphorylated eNOS. SCF levels are down-regulated, and the LTR-HSC pool is decreased to the benefit of the enlarged progenitor pool. How the microenvironment (ME) itself is influenced by the lack of SDF-1 is yet to be defined. (B) A suggested model for the regulation of hematopoiesis by SDF-1 in steady state and after 5-FU myeloablation. (Left) During steady state, SDF-1–depleted mice have decreased levels of LTR-HSCs probably due to reduced self-renewal. In addition, these mice possess an enlarged proliferating pool of HSPCs with no change in WBC counts. (Right) After 5-FU myeloablation, SDF-1–depleted mice exhibit enhanced recovery of both progenitors and mature WBCs, probably also at the expense of LTR-HSCs. This enhanced hematopoietic recovery provides these mice with a clear survival advantage compared with WT mice. (Professional illustration by Paulette Dennis.)
Previous studies that examined hematopoiesis, under CXCR4 conditional deletion, reported exhaustion of long-term repopulating (LTR) HSC due to their hyperproliferation and loss of quiescence in the hematopoietic stem and progenitor cell (HSPC) populations.5,6 Tzeng et al also observed that under SDF-1 conditional deletion the LTR-HSC population decreased over time. This reduction was accompanied by an increase in more committed progenitor populations in the BM, blood, and spleen. The authors reasoned that the exhaustion of LTR-HSCs is a consequence of hyperproliferation and loss of quiescence among HSPCs. Indeed, HSPCs from SDF-1–deleted mice incorporated more BrdU and were observed to a lesser degree in the G0 stage of cell cycle (see figure).
Most interesting was the observation that HSPCs in the murine spleen expanded more dramatically (5- to 15-fold) in comparison with the BM (only 2- to 3-fold) in the absence of SDF-1. This finding correlates well to another observation showing that during both the neonatal stage and in adulthood the levels of SDF-1 in the spleen are much higher than in the BM (∼ 30-fold and ∼ 5-fold, respectively).1 Understanding the physiologic significance of these differences observed between the 2 hematopoietic sites requires additional studies. However, it implies that a population of CXCR4+ HSPCs, which resides in the murine spleen in proximity to stromal cells, is predominantly enforced to be quiescent by high SDF-1 levels in the splenic microenvironment. These HSPCs seem to possess a higher expansion potential, relative to BM-residing HSPCs, which can be unleashed in the absence of SDF-1.
Unexpectedly, and unlike in adult CXCR4 conditionally deleted mice, the increased hyperproliferation under SDF-1 deficiency provided the mice with the advantage of improved recovery and increased survival after 5-FU inflicted myeloablative stress. This can be explained by the fact that these mice maintain an expanded pool of proliferating HSPC with increased differentiation potential which can replenish the mature white blood cells (WBC) pool as evident by higher WBC counts and increased BM cellularity 7 and 14 days after 5-FU treatment (see figure).
Because SDF-1 is presented mainly by the mesenchymal stromal compartment in the BM, the authors hypothesized that the observed hematopoietic phenotypes are driven by the SDF-1–deficient stromal cells. To segregate between these 2 compartments and to study how the SDF-1–lacking stromal microenvironment influences hematopoiesis, in vitro cocultures and in vivo chimeric mice were established. These models proved that in the absence of SDF-1, the stromal compartment fails to maintain LTR-HSC in vitro and facilitates in vivo a hyperproliferative state of cells transplanted from wild-type (WT) mice. Importantly, stromal-expressed cytokines which regulate HSC such as stem cell factor (SCF) are reduced in the absence of SDF-1. The decreased levels of SCF in SDF-1–deleted mice may explain the observed failure to maintain LTR-HSC during HSPC hyperproliferation, as these mice exhibit a phenotype similar to c-Kit (SCF receptor) deficient mice. It may also explain the preferential post–5-FU recovery of the more committed progenitors and mature WBC at the expense of LTR-HSC. Yet, this aspect still needs further investigation to reveal how the stromal microenvironment itself is influenced by the lack of SDF-1, as it was previously shown that both murine and human BM stromal cells functionally express CXCR4.2,7 Previous studies established that SLAM cells (representing LTR-HSC) interact with BM reticular adventitial cells expressing high levels of CXCL12 (CAR cells).6 More recently, a subset of these cells, positive for the neurofilament Nestin and expressing the highest levels of SDF-1, were identified as mesenchymal stem cells (MSCs), regulating HSC homing and maintenance.8 Applying conditional deletion of the SDF-1 allele from different HSC-supportive stromal cell types will reveal the roles of this ligand in regulation of HSCs, MSCs, and various stromal cell types in the BM and spleen (see figure).
Tzeng and colleagues also observed that in the absence of SDF-1, there is a significant topographic change in the BM in terms of localization of hematopoietic sites. During steady-state conditions, in the absence of SDF-1, HSPCs were not detected alongside the endosteum region, the border between the bone and the BM which contains high levels of SDF-1 presented by immature bone-lining osteoblasts.2,7 In addition, after 5-FU treatment, hematopoietic recovery was restricted only to the perivascular regions suggesting impaired function of the endosteal niches (see figure). Although the bone phenotype and endosteal topography require further investigations, these findings are fascinating considering the parallel study performed by the authors with regard to lung function in the absence of SDF-1.9 The same SDF-1 conditionally deleted mice exhibit a lung phenotype of increased alveolar air space and vessel hyperdilation accompanied with increased levels of phosphorylated endothelial nitric oxide synthase (eNOS). This observation raises the possibility of an indirect effect on hematopoiesis via eNOS activity. eNOS was shown to support HSPC development and proliferation.10 If eNOS is also up-regulated in BM and spleen vessels upon SDF-1 deletion, it may create an HSPC-preferable microenvironment, which may be the reason for the observed aberrant HSPC localization and hyperproliferation.
The article by Tzeng et al advances the field of hematopoiesis by allowing, for the first time, study of HSCs in their microenvironment under in vivo physiologic conditions in which SDF-1 is deleted during adulthood. The study reveals the importance of SDF-1 not only as a pivotal HSC chemoattractant,2,7 but also as the keeper of HSC quiescence, HSPC pool size, and proper niche functionality in the BM and spleen.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal