Abstract
Critically ill patients are at heightened risk for nosocomial infections. The anaphylatoxin C5a impairs phagocytosis by neutrophils. However, the mechanisms by which this occurs and the relevance for acquisition of nosocomial infection remain undetermined. We aimed to characterize mechanisms by which C5a inhibits phagocytosis in vitro and in critically ill patients, and to define the relationship between C5a-mediated dysfunction and acquisition of nosocomial infection. In healthy human neutrophils, C5a significantly inhibited RhoA activation, preventing actin polymerization and phagocytosis. RhoA inhibition was mediated by PI3Kδ. The effects on RhoA, actin, and phagocytosis were fully reversed by GM-CSF. Parallel observations were made in neutrophils from critically ill patients, that is, impaired phagocytosis was associated with inhibition of RhoA and actin polymerization, and reversed by GM-CSF. Among a cohort of 60 critically ill patients, C5a-mediated neutrophil dysfunction (as determined by reduced CD88 expression) was a strong predictor for subsequent acquisition of nosocomial infection (relative risk, 5.8; 95% confidence interval, 1.5-22; P = .0007), and remained independent of time effects as assessed by survival analysis (hazard ratio, 5.0; 95% confidence interval, 1.3-8.3; P = .01). In conclusion, this study provides new insight into the mechanisms underlying immunocompromise in critical illness and suggests novel avenues for therapy and prevention of nosocomial infection.
Introduction
The systemic inflammatory response syndrome (SIRS) is classically characterized by profound immune activation, accompanying massive cytokinemia and organ damage.1,2 However, SIRS is accompanied by a counter-regulatory immune suppression sometimes termed the compensatory anti-inflammatory response syndrome (CARS).3 This relative immune suppression is considered important for effective resolution of inflammation but may extend to maladaptive counter-regulatory anti-inflammatory responses.4,5
The consequences of impaired immune function include enhanced susceptibility to nosocomial infection6 or death from sepsis.7 Neutrophils are the major front-line cellular defense against bacterial pathogens, and acquired defects in neutrophil function have been identified in both animal and human sepsis8,9 as well as sterile SIRS.10,11 However, the mediators driving these defects, and the mechanisms involved, remain uncertain.
Animal studies have implicated uncontrolled activation of the complement system in the pathogenesis of sepsis and sterile SIRS.12-14 The key components mediating vasodilatation and vascular leak—the hallmarks of septic shock—are the anaphylatoxins. These are activated forms of complement factors 3 (C3a) and 5 (C5a).14,15 Animal models of sepsis have also implicated C5a in neutrophil dysfunction.8 Because of the rapid clearance (2- to 3-minute half life) of C5a from the circulation, measurement of plasma concentrations gives an imprecise account of neutrophil exposure to this molecule.16,17 However, when C5a binds to its predominant receptor CD88, the receptor is internalized and surface expression decreased17 with a strong inverse correlation to exposure, so acting as an effective proxy marker for C5a exposure.5,18
We previously demonstrated that profound impairment of phagocytosis by neutrophils was mediated by C5a in a cohort of patients with clinically suspected ventilator-associated pneumonia (VAP).5 However, as this was studied only at a single time point at the time of diagnosis of suspected VAP, it was not possible to determine whether the neutrophil defect was causally related to the development of VAP or merely an epiphenomenon. The uncertainty over the time course and lack of serial measures of neutrophil function has been an issue in previous studies in humans.19
The current study therefore aimed to characterize the mechanism(s) by which C5a impairs phagocytosis by neutrophils, and to determine whether C5a-mediated neutrophil dysfunction predicts the acquisition of nosocomial infection in critically ill patients. We hypothesized that C5a drives a defect in phagocytosis by inhibiting RhoA (the key mediator of actin polymerization in complement-facilitated phagocytosis) and that similar defects would be seen in neutrophils from critically ill patients. Second, we hypothesized that among patients admitted to the intensive care unit (ICU), those with C5a-mediated neutrophil dysfunction would go on to acquire a higher rate of nosocomial infection than those without this defect.
Methods
Reagents
Recombinant human C5a (rhC5a), formyl methionyl leucyl phenylalanine (fMLF), and fluorescein isothiocyanate (FITC)–labeled phalloidin were obtained from Sigma-Aldrich. Recombinant human granulocyte-macrophage colony stimulating factor (GM-CSF) was from Affinity Bioreagents. Fluorophore-labeled antibodies were from Invitrogen—the following antibody/fluorophore combinations were used: anti–human CD11b/FITC; anti–human CD62L (L-selectin)/tricolor (TC); anti–human CD16/TC; and anti–human CD64-R-phycoeyrthrin (RPE). Anti–human CD88/Alexa647 was from AbD Serotec. Specific inhibitors of the γ (AS605240) and δ (IC87114) isoforms of phosphoinositide-3-kinase (PI3K) were obtained from Tocris Bioscience and Symansis, respectively. The non–isoform-specific PI3K inhibitor LY294002 and -specific PI3K activator 740Y-P were obtained from Tocris Bioscience. Enzyme-linked immunosorbent assays (ELISAs) for the small Rho GTPases, RhoA, RAC, and CDC42 were obtained from Cytoskeleton.
Isolation of neutrophils
For experiments studying phagocytosis, Rho GTPase, and actin, purified human neutrophils were used. Neutrophils were extracted from heparinized whole blood by centrifugation over proprietary neutrophil separation media (Polymorphoprep; Axis-Shield). Only samples yielding > 95% neutrophil purity were used in experiments (as assessed by Reastain Diff-quik [Reagena] staining of cytospins and analysis using a hemacytometer).
Phagocytosis assay
Neutrophils were adhered to tissue culture plastic in Iscove modified Dulbecco medium (IMDM) containing 1% autologous serum. Cells were exposed for 1 hour to zymosan particles (Sigma-Aldrich) that had been preincubated with 50% autologous serum. Neutrophils were air dried, fixed with methanol, and stained with Reastain Diff-Quik. Light microscopy was used to distinguish neutrophils containing ≥ 2 zymosan particles.5 Duplicate counts were performed on 4 randomly selected fields (minimum of 100 neutrophils per field).
The time course of phagocytosis was explored by exposing cells to zymosan for 5, 10, 15, 30, and 60 minutes, and “velocity” calculated by change in the percentage of neutrophils undergoing phagocytosis over time. To examine the role of C5a, healthy neutrophils were exposed to 100nM rhC5a for 1 hour before zymosan exposure. To explore the role of PI3K, cells were incubated with 6.6μM LY294002 (non–isoform-specific PI3K inhibitor), 8nM AS605240 (the γ isoform inhibitor), or 180nM IC87114 (the δ isoform inhibitor) for 15 minutes before C5a exposure, the doses being the IC50s for these agents.20-22 The effect of GM-CSF was examined by exposing C5a-treated cells to 0.3nM (5 ng/mL) GM-CSF for 30 minutes before adding zymosan.
Photomicrographs were obtained using an inverted light microscope (Axiovert S100, Zeiss) using the ×32 LD A-plan lens. Cells were fixed and mounted on tissue culture plates (Corning Costar), and imaged at room temperature. Images were captured using a Coolsnap camera (RS Photometrics) and Openlab software (Version 5; Perkin Elmer) with no further image processing.
RhoA analysis
For Rho GTPase analysis, neutrophils were adhered to plastic cell culture plates and treated as described before being exposed to zymosan or vehicle control for 5 minutes (previously established as the time of peak Rho activation), before being washed in ice-cold phosphate-buffered saline (PBS) and then lysed with cell lysis solution (Cytoskeleton) containing 10× HALT protease and phosphatase inhibitor with 5mM ethylene diamine tetra-acetic acid (EDTA; Thermo Scientific). Aliquots were taken for protein estimation with the remainder being snap frozen in liquid nitrogen and stored at −80°C before further use. Analysis of samples, equalized for total protein concentration, was by enzyme-linked immunosorbent assay (ELISA; Rho G-lisa, Cytoskeleton), as per the manufacturer's instructions.
Actin polymerization
Neutrophils were adhered and treated as described, before being exposed to vehicle control or zymosan for 5, 10, 15, or 30 minutes. Cells were washed in ice-cold PBS before being fixed in 10% formalin for 10 minutes, permeabilized in 0.1% Triton for 3 minutes, washed in PBS and then incubated with FITC-conjugated phalloidin (2 μg/mL), which irreversibly binds to polymerized filamentous actin (f-actin) but not to nonpolymerized actin, for 30 minutes. Cells were washed once more before being suspended in PBS. Analysis of fluorescence was by flow cytometry (FACScan; BD Biosciences).
Transmigration assay
Neutrophils from healthy volunteers were subjected to various treatments detailed at the end of this section, washed twice, and then added to polystyrene inserts (Corning Costar) containing pores of 3-mm diameter, at 100 000 cells per insert. The inserts were in turn placed in 24-well plates containing (1) IMDM and fMLF as a positive control for migration (final concentration 100nM), or (2) IMDM alone (as negative control). Plates were incubated at 37°C for 1 hour. The upper surface of each insert was gently scraped to remove adherent neutrophils, then inserts were fixed in methanol and then stained with Diff-Quik. Transmigration was quantified by counting the number of neutrophils on the lower surface of the insert, counting 10 randomly selected fields per insert. The pretreatments were as follows: (1) vehicle control, (2) 180nM IC87114 for 15 minutes, (3) 30μM 740 Y-P for 1 hour, (4) 100nM C5a for 1 hour, (5) 180nM IC87114 for 15 minutes followed by 100nM C5a for 1 hour, (6) 100nM C5a for 1 hour followed by 0.3nM GM-CSF for 30 minutes.
Flow cytometry
A total of 50 μL EDTA-anticoagulated whole blood was incubated with primary, fluorophore-tagged antibodies described in “Reagents,” or isotype controls, for 30 minutes at 4°C. Cells were fixed with FACS Lyse (BD Bioscience) and analyzed by FACSCalibur (BD Biosciences), gating on neutrophils by forward/side scatter characteristics. Expression was quantified by the geometric mean channel fluorescence (GMF), which is expressed in arbitrary fluorescence units. The effect of C5a exposure on neutrophil CD88 expression was explored by incubating healthy donor neutrophils with varying concentrations of rhC5a (1nM, 10nM, 100nM, and vehicle control) followed by incubation with the fluorophore-labeled anti-CD88 antibody.
Neutrophil dysfunction
We used neutrophil CD88 as a marker of C5a-mediated neutrophil dysfunction.5 CD88 is the receptor for C5a, and healthy neutrophil CD88 levels show a strong inverse correlation with exposure to varying concentrations of rhC5a (supplemental Methods and Results, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). A cutoff GMF of 250 arbitrary fluorescence units was used to dichotomize patients into those with and without neutrophil dysfunction. The rationale and justification for selecting this cutoff are presented in supplemental Results. Patients were categorized by the sample taken most proximally to an end point (death, infection, or discharge without infection), although in the case of those acquiring infection, neutrophil CD88 was censored for 2 days before the diagnosis of infection to reduce the risk that observed CD88 levels might reflect the presence of new infection. Patients were also analyzed to determine whether their neutrophil dysfunction status changed during admission.
Anaphylatoxin and cytokine assays
Concentrations of C3a were determined by ELISA (OptEIA, BD Biosciences) as per the manufacturer's instructions. Assays were performed using plasma from EDTA/Futhan anti-coagulated blood to reduce the risk of in vitro complement activation.23 Concentrations of IL-1beta, IL-6, IL-8, IL-10, and tumor necrosis factor α (TNF-α) were determined by cytometric bead array (BD Biosciences). Data were analyzed for first and last sample (censored for 2 days before event in those patients acquiring an infection in ICU) to evaluate change in anaphylatoxin/cytokine level during admission.
Volunteers, patients, and setting
Healthy volunteers were recruited from University of Edinburgh staff.
The clinical study took place in an 18-bed teaching hospital medical-surgical ICU that admits approximately 1000 patients per annum from all specialties with the exception of cardiac surgery and isolated neuro-trauma.
Critically ill patients, defined as those admitted to ICU and requiring exogenous support of one or more organ systems (invasive ventilation, inotropes, or hemofiltration) and predicted to require such support for 48 hours or more, were screened for recruitment. Exclusion criteria were age < 16; pregnancy; known HIV infection; known in-born errors of neutrophil metabolism; hematologic malignancy; use of immunosuppressive drugs other than corticosteroids; and unlikelihood of surviving for more than 24 hours. Informed consent was obtained directly from patients when possible; otherwise, informed assent was obtained from the next of kin, in accordance with the Declaration of Helsinki. A “study admission” sample of whole blood was collected into heparin or EDTA/Futhan (BD Biosciences) within 48 hours of ICU admission. Subsequent EDTA/Futhan samples were taken at 2 days after ICU admission, then every 2 subsequent days until day 7 and then every 3-4 days until a study end point was achieved. Study end points were ICU-acquired infection (see next section for definition), death without ICU-acquired infection or discharge from ICU without ICU-acquired infection.
Comorbid conditions were defined and scored using the Charlson Index.24
Infections
Diagnostic criteria were predefined for the major ICU-acquired infections, namely VAP, blood stream infection, vascular catheter-related infection, urinary tract infection, and surgical site/soft-tissue infections. These are based on the definitions used by the Hospitals in Europe Link for Infection Control by Surveillance System (HELICS).25 Where infection was strongly clinically suspected but did not fulfill HELICS criteria (for instance, when cultures were taken while on antibiotics and/or cultures were negative/equivocal), patients were reviewed by an expert panel and the presence or absence of infection adjudicated. The adjudication outcome could be “confirmed,” “probable,” or “unlikely” infection. Details of diagnostic criteria and expert panel adjudication procedures are set out in supplemental Methods.
Statistical analysis
Analysis was conducted using Prism Version 4.0 (GraphPad Software). Comparisons between groups were made using the Mann-Whitney U test for 2 unpaired conditions, Wilcoxon rank sum test for paired data, and the Kruskal-Wallis test for > 2 conditions, using the Dunn posthoc analysis test. Where 2 variables (eg, time and treatment) were present, analysis was undertaken by 2-way ANOVA with posthoc analysis by Bonferroni test. Correlations were assessed by Spearman rho and regression by linear regression. Categorical data were assessed by χ2 test. Analysis of time to infection, censored for death or discharge, was conducted using the log-rank test. P = .05 was considered statistically significant.
Ethical approval
The study was approved by the relevant research ethics committees (RECs). The work on critically ill patients was approved the Scotland A REC (study number 09/MRE00/19) and healthy volunteers by Lothian REC (study number 08/S1103/38).
Results
Studies using neutrophils from healthy volunteers
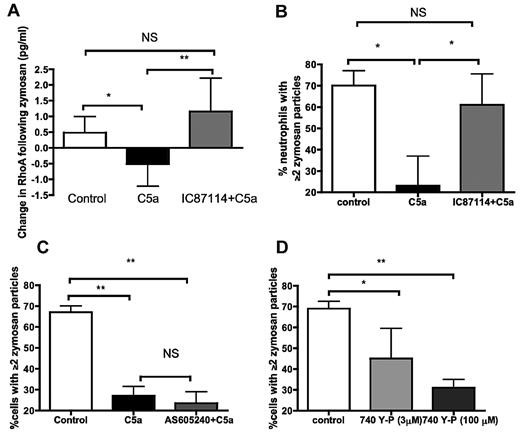
In keeping with our previous work,5 recombinant human C5a (rhC5a) was able to impair phagocytosis of zymosan by neutrophils from healthy volunteers (supplemental Figure 1). As RhoA is the key mediator of actin polymerization during complement-mediated phagocytosis,26 the process involved in ingestion of serum-opsonized zymosan, the effect of C5a treatment on zymosan-induced RhoA activation was examined. C5a prevented the activation of RhoA seen in control-treated neutrophils (Figure 1A first 2 columns). Alternative Rho GTPases (RAC and CDC42) were not inhibited by C5a (median change in RAC, 0.13 pg/mL in control vs 0.16 pg/mL in C5a-treated cells, P = 1 by Mann-Whitney; median change in CDC42, 0.01 pg/mL in control vs −0.02 pg/mL in C5a-treated cells, P = 1 by Mann-Whitney).
C5a inhibits RhoA activation during phagocytosis by neutrophils, by a PI3Kδ-dependent effect. (A) C5a inhibits the activation of neutrophil RhoA in response to opsonized zymosan, and preincubation with PI3K inhibitor IC87114 prevents this inhibition. P = .005 by Kruskal-Wallis ANOVA; *P < .05, **P < .01 by Dunn posthoc test. Data presented as median and interquartile ranges (n = 10 healthy donors). (B) The effect of rhC5a on phagocytosis is prevented by preincubation with the PI3Kδ inhibitor IC87114 at 180nM (IC50 for PI3Kδ). P = .008 by Kruskal-Wallis ANOVA; *P < .05 by Dunn posthoc test. Data are shown as median and interquartile range (n = 5 healthy volunteers). (C) The effect of rhC5a on phagocytosis is not prevented by preincubation with the PI3Kγ inhibitor AS605240 at 8nM (IC50 for PI3Kγ). P = .02 by Kruskal-Wallis ANOVA; *P < .05, **P < .01 by Dunn posthoc test. Data are shown as median and interquartile range (n = 5 healthy volunteers). (D) Incubating neutrophils with the PI3KIA (α, β, δ) activator 740Y-P inhibits phagocytosis. P = .01 by Kruskal-Wallis ANOVA; *P < .05, **P < .01 by Dunn posthoc test. Data are shown as median and interquartile range (n = 5 healthy volunteers).
C5a inhibits RhoA activation during phagocytosis by neutrophils, by a PI3Kδ-dependent effect. (A) C5a inhibits the activation of neutrophil RhoA in response to opsonized zymosan, and preincubation with PI3K inhibitor IC87114 prevents this inhibition. P = .005 by Kruskal-Wallis ANOVA; *P < .05, **P < .01 by Dunn posthoc test. Data presented as median and interquartile ranges (n = 10 healthy donors). (B) The effect of rhC5a on phagocytosis is prevented by preincubation with the PI3Kδ inhibitor IC87114 at 180nM (IC50 for PI3Kδ). P = .008 by Kruskal-Wallis ANOVA; *P < .05 by Dunn posthoc test. Data are shown as median and interquartile range (n = 5 healthy volunteers). (C) The effect of rhC5a on phagocytosis is not prevented by preincubation with the PI3Kγ inhibitor AS605240 at 8nM (IC50 for PI3Kγ). P = .02 by Kruskal-Wallis ANOVA; *P < .05, **P < .01 by Dunn posthoc test. Data are shown as median and interquartile range (n = 5 healthy volunteers). (D) Incubating neutrophils with the PI3KIA (α, β, δ) activator 740Y-P inhibits phagocytosis. P = .01 by Kruskal-Wallis ANOVA; *P < .05, **P < .01 by Dunn posthoc test. Data are shown as median and interquartile range (n = 5 healthy volunteers).
C5a activates PI3K27 including both γ and δ isoforms,28 and previous incubation of neutrophils with PI3K inhibitors such as LY294002 prevents the effect of C5a on phagocytosis5 (supplemental Figure 2). In cell lines, PI3Kδ has demonstrated RhoA inhibitory effects,29 so to explore whether activation of PI3Kδ could mediate the effect seen with rhC5a we preincubated neutrophils with the PI3Kδ-specific inhibitor IC87114. The final column in Figure 1A demonstrates that this prevented the inhibition of RhoA.
The effect of PI3Kδ inhibition on the ability of C5a to inhibit phagocytosis, as well as RhoA inhibition, was examined by preincubating neutrophils with IC87114 before C5a exposure in phagocytosis experiments. IC87114 was able to prevent C5a-mediated impairment of phagocytosis consistent with its effect on RhoA (Figure 1B). In striking contrast, inhibition of the other major leukocyte PI3K isoform, PI3Kγ, using the isoform-specific inhibitor AS605240, was unable to prevent the effects of C5a (Figure 1C). The pan-isoform inhibitor LY294002, at the IC50 for PI3Kγ, was able to prevent the effect of C5a (supplemental Figure 2), indicating that the failure of PI3Kγ inhibition to restore phagocytosis was not because of the inhibitor blocking phagocytosis itself. Confirmation that AS605240 was functional was obtained by showing that it prevented AKT phosphorylation in response to C5a in neutrophils (supplemental Figure 3). The effect of IC87114 on preventing C5a-mediated phagocytic dysfunction persisted down to the IC25 (60nM; see supplemental Figure 4), a dose that did not fully prevent AKT phosphorylation (see supplemental Figure 5), suggesting that IC87114 was acting selectively on PI3Kδ. Exposure of neutrophils to 740Y-P, a cell-permeable specific activator of the p85 subunit of class IA PI3Ks (ie, PI3Kα, β, and δ)30 was able to reproduce the defect in phagocytosis in a dose-dependent manner (Figure 1D).
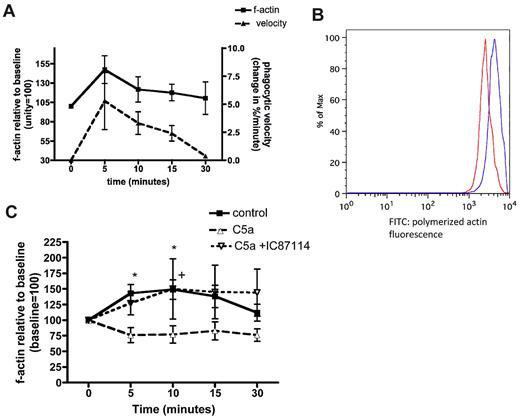
Actin polymerization allows the engulfment of phagocytic targets, and paralysis of this system inhibits phagocytosis.31 The polymerization and depolymerization of actin, as assessed by binding of fluorescent phalloidin, followed the same time course as the “velocity” of phagocytosis in our model (Figure 2A-B). Treatment of healthy volunteer neutrophils with C5a inhibited this polymerization response (Figure 2C). Indeed there was a small reduction in f-actin within neutrophils, consistent with the reduction in active RhoA noted in Figure 1A. Pretreatment with the PI3Kδ inhibitor IC87114 prevented this effect (upper hatched line in Figure 2C), again mirroring the changes in RhoA activation. A similar effect was achieved with the pan PI3K inhibitor LY294002 (see supplemental Figure 6).
The polymerization of actin in response to zymosan. (A) Change in polymerized filamentous (f) actin over time following exposure of neutrophils to zymosan, compared with the time course of phagocytosis. Data shown as mean values from n = 3 healthy donors. (B) Representative histograms illustrating the shift in geometric mean fluorescence before (red) and after (blue) exposure of cells to zymosan, indicating actin polymerization. (C) Change in polymerized f-actin over time in neutrophils treated with vehicle control (■―), rhC5a (▾ ), or IC87114 followed by rhC5a (▵
), or IC87114 followed by rhC5a (▵ ), then exposed to opsonized zymosan. Data shown as mean and SEM, P < .0001 for difference between treatment groups by 2-way ANOVA, *P < .05 between control and C5a treatment, +P < .05 between C5a + IC87114 and C5a treatment by Bonferroni posthoc test (n = 8 healthy donors).
), then exposed to opsonized zymosan. Data shown as mean and SEM, P < .0001 for difference between treatment groups by 2-way ANOVA, *P < .05 between control and C5a treatment, +P < .05 between C5a + IC87114 and C5a treatment by Bonferroni posthoc test (n = 8 healthy donors).
The polymerization of actin in response to zymosan. (A) Change in polymerized filamentous (f) actin over time following exposure of neutrophils to zymosan, compared with the time course of phagocytosis. Data shown as mean values from n = 3 healthy donors. (B) Representative histograms illustrating the shift in geometric mean fluorescence before (red) and after (blue) exposure of cells to zymosan, indicating actin polymerization. (C) Change in polymerized f-actin over time in neutrophils treated with vehicle control (■―), rhC5a (▾ ), or IC87114 followed by rhC5a (▵
), or IC87114 followed by rhC5a (▵ ), then exposed to opsonized zymosan. Data shown as mean and SEM, P < .0001 for difference between treatment groups by 2-way ANOVA, *P < .05 between control and C5a treatment, +P < .05 between C5a + IC87114 and C5a treatment by Bonferroni posthoc test (n = 8 healthy donors).
), then exposed to opsonized zymosan. Data shown as mean and SEM, P < .0001 for difference between treatment groups by 2-way ANOVA, *P < .05 between control and C5a treatment, +P < .05 between C5a + IC87114 and C5a treatment by Bonferroni posthoc test (n = 8 healthy donors).
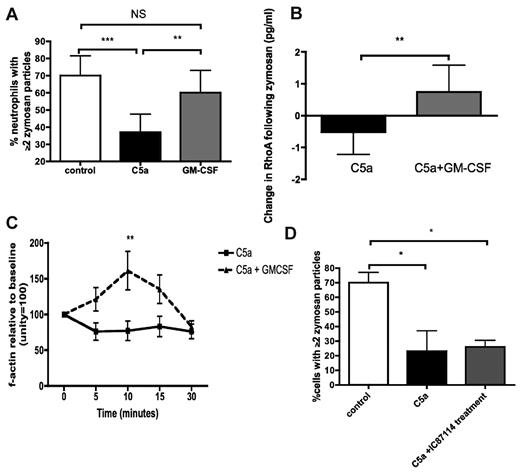
To examine the potential for therapeutic intervention, we explored the ability of GM-CSF to reverse the defects noted above. GM-CSF, applied after 30 minutes of C5a treatment, was able to restore phagocytic function in healthy neutrophils (Figure 3A). GM-CSF treatment was also able to resurrect RhoA activation and actin polymerization in C5a-treated cells (Figure 3B and C, respectively). In contrast to pretreatment with IC87114 (Figure 1B), application of IC87114 after C5a was unable to resurrect phagocytosis (Figure 3D), suggesting that GM-CSF operates via a PI3Kδ-independent pathway.
GM-CSF reverses the defects in phagocytosis induced by rhC5a. (A) Addition of GM-CSF restores effective phagocytosis of opsonized zymosan in neutrophils exposed to C5a. P < .0001 by Kruskal-Wallis; **P < .01, ***P < .001 by Dunn posthoc test. Data are displayed as median and interquartile range (n = 10 healthy donors). (B) Addition of GM-CSF restores RhoA activation in neutrophils treated with rhC5a and exposed to opsonized zymosan. **P = .007 by Mann-Whitney U test. Data are displayed as median and interquartile range (n = 10 healthy donors). Note: C5a data are the same as those presented in Figure 1A, and the GM-CSF data are shown separately for reasons of clarity—separation does not alter the statistical significance of the differences noted. (C) Addition of GM-CSF restores actin polymerization in neutrophils treated with rhC5a and exposed to opsonized zymosan. P = .0001 for differences between treatments by 2-way ANOVA, **P < .01 by Bonferroni posthoc test for differences between treatments. Data are shown as mean and SEM (n = 8 healthy donors). (D) Addition of PI3Kδ inhibitor IC87114 after 30 minutes of treatment with C5a fails to resurrect phagocytosis. P = .009 by Kruskal-Wallis, *P < .05 by Dunn posthoc test. Data are displayed as median and interquartile range (n = 5 healthy donors).
GM-CSF reverses the defects in phagocytosis induced by rhC5a. (A) Addition of GM-CSF restores effective phagocytosis of opsonized zymosan in neutrophils exposed to C5a. P < .0001 by Kruskal-Wallis; **P < .01, ***P < .001 by Dunn posthoc test. Data are displayed as median and interquartile range (n = 10 healthy donors). (B) Addition of GM-CSF restores RhoA activation in neutrophils treated with rhC5a and exposed to opsonized zymosan. **P = .007 by Mann-Whitney U test. Data are displayed as median and interquartile range (n = 10 healthy donors). Note: C5a data are the same as those presented in Figure 1A, and the GM-CSF data are shown separately for reasons of clarity—separation does not alter the statistical significance of the differences noted. (C) Addition of GM-CSF restores actin polymerization in neutrophils treated with rhC5a and exposed to opsonized zymosan. P = .0001 for differences between treatments by 2-way ANOVA, **P < .01 by Bonferroni posthoc test for differences between treatments. Data are shown as mean and SEM (n = 8 healthy donors). (D) Addition of PI3Kδ inhibitor IC87114 after 30 minutes of treatment with C5a fails to resurrect phagocytosis. P = .009 by Kruskal-Wallis, *P < .05 by Dunn posthoc test. Data are displayed as median and interquartile range (n = 5 healthy donors).
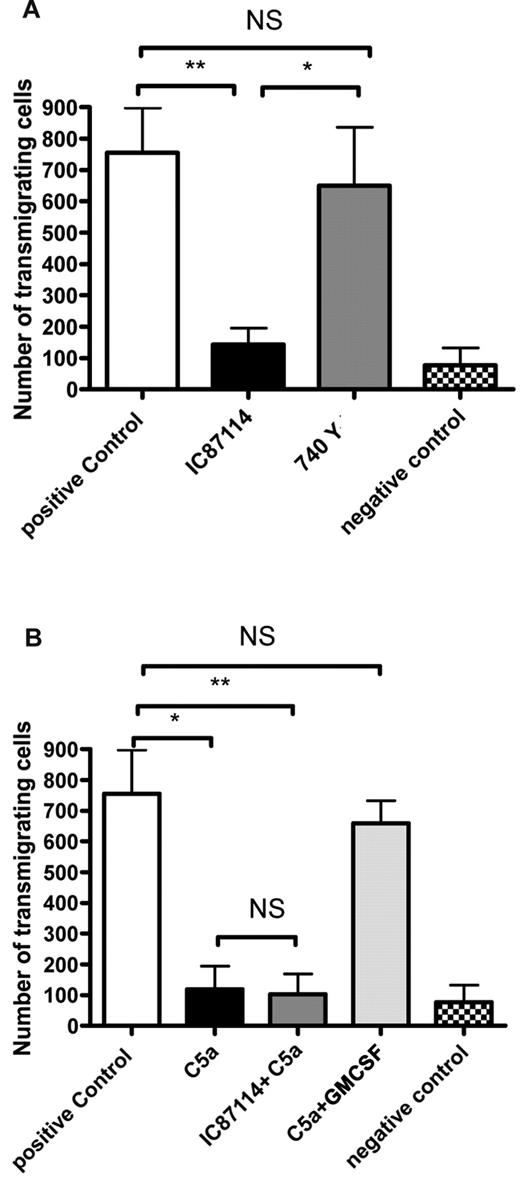
To examine whether PI3Kδ is central to other neutrophil dysfunctions induced by C5a, the effects of the inhibitor (IC87114) and activator (740Y-P) on transmigration were assessed. In contrast to phagocytosis, IC87114 alone inhibited transmigration and 740 Y-P had no inhibitory effect (Figure 4A). Unsurprisingly, IC87114 was unable to prevent the defect in transmigration induced by C5a; however, treatment with GM-CSF was (Figure 4B) again pointing to GM-CSF operating via PI3Kδ-independent pathways.
The effects of C5a and PI3Kdelta modulation on neutrophil transmigration. (A) Transmigration by neutrophils across a 3-μg transwell membrane toward an fMLF target following pretreatment with vehicle control, 180nM IC87114 or 30μM 740 Y-P. Negative control is without fMLF. P = .0029 by Kruskal-Wallis; *P < .05, **P < .01, NS P > .05 by Dunn posthoc test. (B) Transmigration by neutrophils across a 3-μg transwell membrane toward an fMLF target following pretreatment with vehicle control, 100nM C5a, 180nM IC87114 followed by 100nM C5a, or 100nM C5a followed by 0.3nM GM-CSF. Negative control is without fMLF. P = .0005 by Kruskal-Wallis; *P < .05, **P < .01, NS P > .05 by Dunn posthoc test.
The effects of C5a and PI3Kdelta modulation on neutrophil transmigration. (A) Transmigration by neutrophils across a 3-μg transwell membrane toward an fMLF target following pretreatment with vehicle control, 180nM IC87114 or 30μM 740 Y-P. Negative control is without fMLF. P = .0029 by Kruskal-Wallis; *P < .05, **P < .01, NS P > .05 by Dunn posthoc test. (B) Transmigration by neutrophils across a 3-μg transwell membrane toward an fMLF target following pretreatment with vehicle control, 100nM C5a, 180nM IC87114 followed by 100nM C5a, or 100nM C5a followed by 0.3nM GM-CSF. Negative control is without fMLF. P = .0005 by Kruskal-Wallis; *P < .05, **P < .01, NS P > .05 by Dunn posthoc test.
Studies using neutrophils from critically ill patients
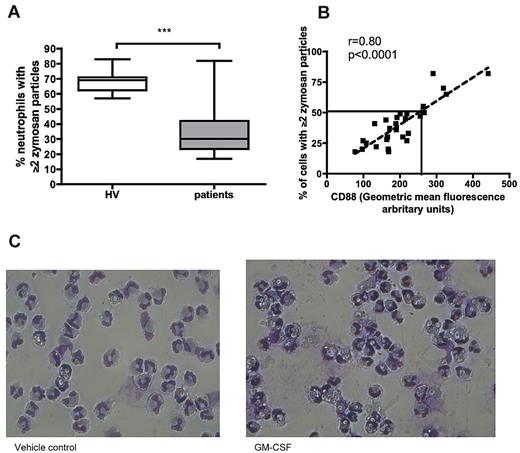
Blood samples were taken from a cohort of critically ill patients at several time points. Neutrophils from samples taken at study entry (within 48 hours of ICU admission) were assessed for phagocytosis. Phagocytosis by neutrophils from critically ill patients was profoundly impaired (Figure 5A,C). Similar to our findings in patients with suspected VAP (who had been in ICU for a median of 8 days),5 CD88 correlated strongly with phagocytosis in patients newly admitted to ICU (Figure 5B). In contrast, other classic markers of neutrophil activation, namely CD11b, CD16, CD64, and CD62L, showed no significant correlation with phagocytosis (r = −0.05, 0.12, 0.15, 0.01, respectively, all P > .05 by Spearman rho), suggesting that the relationship observed for C5a/CD88 does not simply reflect generalized neutrophil activation.
Phagocytosis by neutrophils from critically ill patients is defective and related to C5a exposure. (A) Phagocytosis by patients' neutrophils is significantly reduced compared with healthy volunteers. ***P = .001 by Mann-Whitney U test. Data are shown as median (central line), interquartile range (box), and range (whiskers) (n = 39 participants: 29 patients and 10 healthy volunteers). (B) Relationship between surface CD88 expression and phagocytosis by neutrophils. P < .0001, r = 0.8 by Spearman rho. The solid line indicates phagocytosis predicted by linear regression at a CD88 of 250 GMF units, the predefined cutoff for neutrophil dysfunction (n = 29 patients). (C) Representative images of patient neutrophils exposed to zymosan before (left) and after (right) GM-CSF treatment (magnification ×32).
Phagocytosis by neutrophils from critically ill patients is defective and related to C5a exposure. (A) Phagocytosis by patients' neutrophils is significantly reduced compared with healthy volunteers. ***P = .001 by Mann-Whitney U test. Data are shown as median (central line), interquartile range (box), and range (whiskers) (n = 39 participants: 29 patients and 10 healthy volunteers). (B) Relationship between surface CD88 expression and phagocytosis by neutrophils. P < .0001, r = 0.8 by Spearman rho. The solid line indicates phagocytosis predicted by linear regression at a CD88 of 250 GMF units, the predefined cutoff for neutrophil dysfunction (n = 29 patients). (C) Representative images of patient neutrophils exposed to zymosan before (left) and after (right) GM-CSF treatment (magnification ×32).
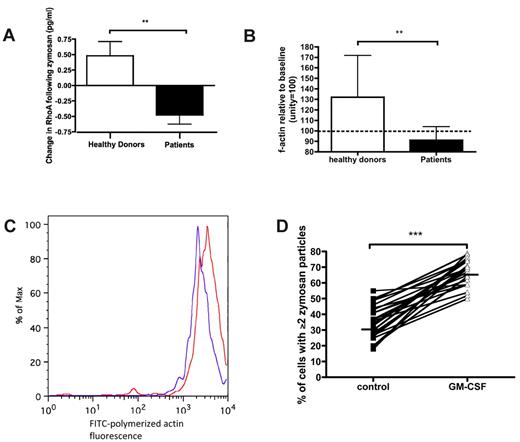
We proceeded to evaluate similarities between the mechanistic features determined during exposure of healthy neutrophils to rhC5a and those in neutrophils from critically ill patients. Patients' neutrophils exposed to zymosan failed to activate RhoA (Figure 6A). Indeed, a small reduction was observed in RhoA relative to baseline (Figure 6A). Patients' neutrophils were also unable to effectively polymerize actin in response to zymosan (Figure 6B-C). These 2 effects (on RhoA and actin) were in keeping with the effects described for healthy neutrophils exposed to C5a in Figures 1A and 2C. Ex vivo application of GM-CSF was able to restore phagocytosis to levels similar to those seen in healthy volunteers (Figure 6D).
Neutrophils from critically ill patients show similarities to healthy neutrophils that have been exposed to rhC5a. (A) RhoA activation in response to zymosan is impaired in patient neutrophils. **P = .002 by Mann-Whitney U test. Data are shown as median and interquartile range (n = 20: 10 patients and 10 healthy volunteers). (B) Actin polymerization following 5 minutes of zymosan exposure is impaired in patients. **P = .006 by t test. Data are shown as mean and SEM (n = 15: 10 patients and 5 healthy volunteers). (C) Representative histograms illustrating the shift in geometric mean fluorescence before (red) and after (blue) exposure of cells to zymosan; note the contrast with Figure 2B. (D) GM-CSF restores phagocytosis in patients' neutrophils. ***P < .001 by Wilcoxon rank sum test. Solid lines indicate median values (n = 24 patients).
Neutrophils from critically ill patients show similarities to healthy neutrophils that have been exposed to rhC5a. (A) RhoA activation in response to zymosan is impaired in patient neutrophils. **P = .002 by Mann-Whitney U test. Data are shown as median and interquartile range (n = 20: 10 patients and 10 healthy volunteers). (B) Actin polymerization following 5 minutes of zymosan exposure is impaired in patients. **P = .006 by t test. Data are shown as mean and SEM (n = 15: 10 patients and 5 healthy volunteers). (C) Representative histograms illustrating the shift in geometric mean fluorescence before (red) and after (blue) exposure of cells to zymosan; note the contrast with Figure 2B. (D) GM-CSF restores phagocytosis in patients' neutrophils. ***P < .001 by Wilcoxon rank sum test. Solid lines indicate median values (n = 24 patients).
Relationship between C5a-mediated neutrophil dysfunction and acquisition of infection in ICU
Sixty patients who met our definition of critical illness were recruited and serial neutrophil CD88 levels determined. Twenty-two patients (37%) acquired a new infection while in ICU (17 confirmed and 5 probable). The distribution of infections is shown in the supplemental Table 1.
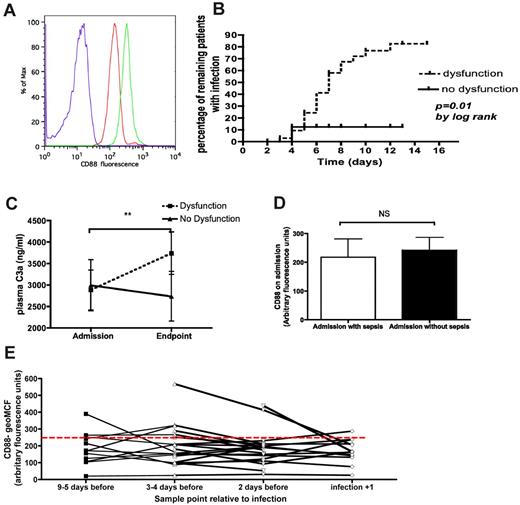
Thirty-eight patients (63%) fell into the “C5a-mediated neutrophil dysfunction” (ie, low CD88) group, while the remaining 22 (37%) were classified as having no dysfunction (ie, high CD88). Forty-eight (80%) of patients remained in the same group as their admission sample, whereas 15% progressed from “no dysfunction” to “dysfunction” and 5% went in the opposite direction. Of the minority who changed groups, all but 3 were in their eventual group by day 3 after admission. Figure 7A shows example flow cytometric histograms for surface CD88 expression on patient and healthy donor neutrophils.
The relationship between CD88 levels and infection in patients. (A) Representative histograms showing fluorescence of healthy donor (green) or patient (red) neutrophils stained with anti-CD88:Alexa647, or isotype controls (blue). (B) Kaplan-Meier plot showing acquisition of infection as a function of time and patients alive while remaining within the ICU. Patients were censored for death or discharge without ICU-acquired infection, P = .01 by log-rank test. (B) Representative histograms showing fluorescence of healthy donor (green) or patient (red) neutrophils stained with anti-CD88:Alexa647, or isotype controls (blue). (C) The change in C3a concentrations over time, comparing the initial sample with the last sample before an end point (discharge, death, or infection) occurred. In the case of infection the “last sample” was censored for 2 days prior to infection. **P = .003 by Wilcoxon rank sum test for the difference between initial and end-point samples for patients with dysfunction (ie, low neutrophil CD88); n = 58 patients. (D) Neutrophil surface CD88 expression on the initial sample from patients admitted to ICU with and without sepsis. NS, P = .59 by Mann-Whitney U test. Data are shown as median and interquartile range (n = 60 patients, 23 with sepsis and 37 without). (E) Neutrophil surface CD88 expression before and after ICU-acquired infection. P = .97 by Kruskal-Wallis ANOVA (n = 22 patients).
The relationship between CD88 levels and infection in patients. (A) Representative histograms showing fluorescence of healthy donor (green) or patient (red) neutrophils stained with anti-CD88:Alexa647, or isotype controls (blue). (B) Kaplan-Meier plot showing acquisition of infection as a function of time and patients alive while remaining within the ICU. Patients were censored for death or discharge without ICU-acquired infection, P = .01 by log-rank test. (B) Representative histograms showing fluorescence of healthy donor (green) or patient (red) neutrophils stained with anti-CD88:Alexa647, or isotype controls (blue). (C) The change in C3a concentrations over time, comparing the initial sample with the last sample before an end point (discharge, death, or infection) occurred. In the case of infection the “last sample” was censored for 2 days prior to infection. **P = .003 by Wilcoxon rank sum test for the difference between initial and end-point samples for patients with dysfunction (ie, low neutrophil CD88); n = 58 patients. (D) Neutrophil surface CD88 expression on the initial sample from patients admitted to ICU with and without sepsis. NS, P = .59 by Mann-Whitney U test. Data are shown as median and interquartile range (n = 60 patients, 23 with sepsis and 37 without). (E) Neutrophil surface CD88 expression before and after ICU-acquired infection. P = .97 by Kruskal-Wallis ANOVA (n = 22 patients).
Clinical and demographic features of these groups, including acquisition of nosocomial infection, are shown in Table 1. The group with dysfunction had a relative risk for subsequently developing infection of 5.8 (95% confidence interval [CI] 1.5-22, P = .0007). As a sensitivity analysis, exclusion of those with “probable” infection revealed a relative risk of 5 (95% CI 1.3-20, P = .006). Similarly, excluding those patients whose dysfunction status changed during their stay did not significantly alter the risk of infection (relative risk 4.8, 95% CI 1.2-19, P = .005). To control for the possibility that increased acquisition of infection could simply reflect increasing duration of stay in ICU, an analysis for time to infection was performed by Kaplan-Meier plot. This confirmed a significant difference between the 2 groups (Figure 7B; hazard ratio 5.0, 95% CI 1.3-8.3, P = .01).
Demographic and clinical features of patients with and without C5a-mediated neutrophil dysfunction.
| Variable . | Dysfunction . | No Dysfunction . | P . |
|---|---|---|---|
| No. of patients | 38 | 22 | |
| Median age, y (range) | 58 (20-88) | 62 (26-80) | .9* |
| Male, % | 50% | 64% | .42† |
| Admitted to ICU with sepsis, % | 32% | 45% | .42† |
| Surgical patients, % | 32% | 31% | 1† |
| Receiving corticosteroids, % | 26% | 19% | .75† |
| Comorbid conditions, median (IQR; Charlson index) | 3 (2-5) | 3 (1-4) | .52* |
| Intubated, % | 92% | 91% | 1† |
| With a central venous catheter, % | 82% | 77% | .74† |
| APACHE II score, median (IQR) | 21 (19-29) | 22 (15-29) | .81* |
| Acquiring infection in ICU, % | 53% | 9% | .0007† |
| Variable . | Dysfunction . | No Dysfunction . | P . |
|---|---|---|---|
| No. of patients | 38 | 22 | |
| Median age, y (range) | 58 (20-88) | 62 (26-80) | .9* |
| Male, % | 50% | 64% | .42† |
| Admitted to ICU with sepsis, % | 32% | 45% | .42† |
| Surgical patients, % | 32% | 31% | 1† |
| Receiving corticosteroids, % | 26% | 19% | .75† |
| Comorbid conditions, median (IQR; Charlson index) | 3 (2-5) | 3 (1-4) | .52* |
| Intubated, % | 92% | 91% | 1† |
| With a central venous catheter, % | 82% | 77% | .74† |
| APACHE II score, median (IQR) | 21 (19-29) | 22 (15-29) | .81* |
| Acquiring infection in ICU, % | 53% | 9% | .0007† |
ICU indicates intensive care unit; IQR, interquartile range; and APACHE, Acute Physiology and Chronic Health Evaluation.
Mann-Whitney test.
Fisher exact test.
To examine the accuracy of our predefined cutpoint for CD88 (ie, GMF of 250 units), we constructed a receiver operator characteristic (ROC) curve comparing the sample most temporally related to infection (censored for 2 days prior) with samples from patients who did not develop infection. Using the Youden method,32 this revealed an optimal cutpoint of 246 units, justifying our predefined definition. Regression analysis of the relationship between CD88 and phagocytosis (Figure 5B) showed that a CD88 with a GMF of 250 units equated to a phagocytosis rate of 50%.
Figure 7C confirms the presence of complement activation in patients with neutrophil dysfunction. In contrast, patients in the no dysfunction group showed a trend toward reduced complement activation. This dichotomized pattern was not replicated for any of the cytokines examined, with the levels remaining unchanged or decreasing in both groups for IL-1β, -6, -8, and -10, and TNF-α (data not shown).
In the analysis described in Figure 7B, we censored patient data at 2 days before infection developing (so as to obviate confounding by infection itself). We further studied the relationship between CD88 and infection by analyzing neutrophil CD88 in patients with and without sepsis at the time of arrival in ICU (Figure 7D). No significant difference was observed between the 2 groups, suggesting that infection itself was not responsible for differences in surface levels of neutrophil CD88. Furthermore, analysis of neutrophil CD88 before and after acquisition of infection in the ICU revealed (1) that CD88 expression was unaffected by the acquisition of infection and (2) that the overwhelming majority of patients had persistently low CD88 expression in the days preceding acquisition of infection (Figure 7E).
Discussion
Our previous work on C5a-mediated human neutrophil dysfunction raised several questions,5 which were the focus of the present study. First, how does C5a, a mediator classically thought to facilitate inflammation, lead to inhibited phagocytosis? Second, does the impairment of phagocytosis in patients bear more than superficial similarity to that induced in healthy neutrophils by rhC5a in vitro? Third, is C5a-mediated dysfunction prevalent in a wider group of critically ill patients and does it have any clinical consequence?
The current study proposes a mechanism by which C5a inhibits phagocytosis by neutrophils. We have demonstrated for the first time that RhoA, a key signaling molecule in actin polymerization in a variety of settings,33 is inhibited by C5a at concentrations found in diseases such as sepsis.14,34 This inhibition of RhoA leads to downstream inhibition of actin polymerization, which then prevents phagocytosis. Interestingly, Kamanova et al showed that RhoA could also be inhibited by cyclic adenosine monophosphate (cAMP).35 Beta-agonists, in contrast to C5a, mediate their inhibition of phagocytosis via cAMP,5,36 raising the possibility of a final common pathway through which these agents (β-agonists and C5a) act.
The identification of PI3Kδ as the upstream mediator of C5a-induced RhoA inhibition is consistent with previous work using different stimuli.26,37 PI3K comprises a broad family of signaling enzymes with diverse structures.38 Of the class I PI3Ks, the IA isoform “δ” and the IB isoform “γ” are largely confined to leukocytes.39 The ability of a PI3Kδ inhibitor, but not a PI3Kγ inhibitor, to prevent C5a-mediated impairment of phagocytosis suggested that our original findings using LY294002 and wortmannin5 were not the result of nonspecific/non-PI3K actions.40 The identified role for PI3Kδ in the specific context of neutrophils/C5a may also explain the apparent paradox whereby PI3Ks have been shown to promote phagocytosis in other settings.41
The final intracellular steps in phagocytosis involve the formation of polymerized actin to facilitate engulfment and phagolysosome formation. C5a is able to block this polymerization response, and the ability of IC87114 (and indeed LY294002) to prevent such a blockade provides a link between engagement of CD88 (a receptor with no reported phagocytic ability), PI3Kδ, and phagocytosis. The proposed pathway is summarized schematically in supplemental Figure 7.
It is important to note that whereas C5a exerts its phagocytic inhibitory effect in a PI3Kδ-dependent manner, this may not be the case for other C5a-mediated dysfunctions. In this paper we have confirmed our earlier finding that C5a can inhibit transmigration.5 However, this effect was independent of PI3Kδ.
The dissociation of effects on phagocytosis and transmigration when PI3Kδ is inhibited in C5a-treated neutrophils has several important implications. We have consistently shown that PI3Kδ inhibition increases intracellular RhoA in C5a-treated neutrophils (Figure 1A). It appears that transmigration relies on a delicate balance of intracellular RhoA levels. Whereas inhibition impairs transmigration,42 heightened levels can impair rather than promote transmigration.43 In addition, other investigators report a threshold effect of RhoA, where only high concentrations of RhoA inhibitor impaired transmigration.44 Furthermore, the effects of PI3Kδ inhibition on neutrophil transmigration do not appear to act through impairment of actin polymerization.45 In contrast, we found that actin polymerization was central to the effects of PI3Kδ inhibition on phagocytosis. It therefore seems plausible that PI3Kδ inhibition restores sufficient RhoA to allow efficient phagolysosome formation and phagocytosis in C5a-treated neutrophils, but that levels of RhoA are insufficient to restore transmigration (and/or that in dysfunctional neutrophils restoration of transmigration requires PI3Kδ/RhoA-independent pathways).
Interestingly, the dissociated effects of PI3Kδ inhibition on phagocytosis and transmigration in dysfunctional, C5a-treated neutrophils could explain why PI3Kδ inhibitors have not proven beneficial in sepsis.46 Certainly our findings do not suggest a therapeutic place for PI3Kδ or RhoA inhibitors for prevention of infection in critically ill patients. In contrast, the ability of GM-CSF to restore phagocytosis in nonneutropenic critically ill patients has been described previously,47 and we also show here that it effectively restores defective transmigration. GM-CSF's growing safety profile and pluripotent effects48,49 make it a potentially attractive therapy. We demonstrate that it is able to reactivate the RhoA response to zymosan, thereby facilitating actin polymerization and hence phagocytosis. Although we have demonstrated that GM-CSF is unlikely to act as a PI3Kδ inhibitor, the mechanism upstream of RhoA remains uncertain and will be the focus of future work.
Some important and related caveats to our work are worth describing at this point. For example, all studies involving C5a and neutrophils must be considered in the light of the experimental system used. Our work specifically addresses the aberrant effect of excessive concentrations on human neutrophil function, consistent with levels found in sepsis.1 Our in vitro studies therefore used 100nM rhC5a, and are quite distinct from either animal studies50 or studies seeking to study the “physiologic” effects of lower concentrations of C5a (at which a promigratory effect is classic). Our work has not sought specifically to address effects of PI3K inhibition in healthy neutrophils not exposed to C5a. Nevertheless, the data in Figure 4A do provide some insights showing that, in healthy neutrophils not exposed to C5a, PI3Kδ does regulate transmigration. This emphasizes the quite different role for neutrophil signaling pathways in the physiologic and pathologic contexts. There are several other reports in the literature of PI3Kδ inhibition preventing transmigration by healthy human neutrophils or neutrophil-like cell lines,45,51 which are consistent with our findings using IC87114. Interestingly, Papakonstanti and colleagues demonstrated IC87114-mediated impairment of transmigration, while showing the simultaneous increase in RhoA that one would expect from our findings.37
Although we previously demonstrated a relationship between markers of C5a exposure and impaired phagocytosis in neutrophils from patients,5 the underlying intracellular features remained unclear. The present study suggests that the changes parallel those observed when C5a is applied to healthy neutrophils, in that there was a reduction in active RhoA and actin polymerization when patients' neutrophils were exposed to zymosan. Furthermore, ex vivo treatment of patients' neutrophils with GM-CSF showed the same restoration of phagocytosis as seen in healthy neutrophils exposed to C5a.
There was a striking divergence in complement activation between patients with low neutrophil CD88 and those with high CD88, shown in Figure 7C. C3a rose in those patients with low neutrophil CD88, indicating ongoing complement activation and C5a release, whereas C3a fell in those with high CD88. In contrast, the inflammatory cytokines showed a general improvement during admission, suggesting that neutrophil dysfunction is more closely associated with the complement system. These data provide further support for the contention that activated complement in general, and C5a specifically, is a major mediator of the defects seen in critically ill patients, and may go some way in explaining the apparent paradox of immune dysfunction in the context of hyperinflammatory conditions such as sepsis and SIRS.
A potential issue with this study relates to the relevance of the assay used to assess phagocytosis. Zymosan particles are derived from yeast (Saccharomyces cerevisiae) and are large compared with other phagocytic targets such as bacteria. Furthermore, the use of cells adhered onto tissue culture plastic in tissue culture medium may not fully reflect the physiologic and pathophysiologic situations found in healthy and diseased humans. As such there remained a risk that our observations reflect in vitro artefacts specific to the model used. Therefore, to test for biologic and clinical relevance, we undertook the third part of this study, examining the relationship between C5a-mediated neutrophil dysfunction and a clinically significant outcome—namely, acquisition of nosocomial infection. Patients whose neutrophils displayed a signature of high C5a exposure were 5 times more likely to acquire such infections. This suggests that C5a, which is known to be released in excessive quantities in situations as diverse as sepsis,52 major trauma,53,54 pancreatitis,55 and major surgery,56 may go some way toward explaining why nosocomial infections are so prevalent in the ICU. Animal work has also demonstrated C5a-mediated dysfunction in the context of experimental sepsis8 and trauma.10
Our previous work recruited patients who had been in ICU for a median of 8 days.5 The present study demonstrates that C5a exposure and the consequent defects occur in many patients early on in their admission to ICU (within 48 hours), and it is tempting to speculate that some patients may acquire this before ICU admission. Examination of the trend in infection acquisition over time suggests that those with dysfunctional neutrophils experience a steady, near-linear accumulation of risk so that the risk becomes a function of exposure (ie, time in ICU). In contrast, those without dysfunction appear relatively resistant to infection, even if they remain for some time in ICU.
What stimulates the systemic, apparently uncontrolled activation of complement and release of C5a in critical illness remains uncertain. Studies of conditions with a defined time of insult, such as major trauma57 or cardiac arrest,58 demonstrate an early and rapid increase in complement activation and C5a concentrations. A variety of complement activation mechanisms may be present in critical illness, including coagulation,59 human neutrophil elastase (HNE)–mediated cleavage of IgG to form F(ab′) portions,60 HNE itself,61 and systemic release and circulation of bacterial peptides.62 Whether systemic activation occurs simply because these activators are released in large quantities, or whether there are underlying defects in the complement regulatory mechanisms, remains to be determined.
C5a can inhibit a range of neutrophil functions including transmigration and reactive oxygen species production.5,8,63 Therefore we cannot be certain that the phagocytic defect specifically leads to the increased risk of nosocomial infection observed. Indeed it is likely that multiple defects act synergistically to produce the immunoparalysis that appears to characterize neutrophils in such severely ill patients. Furthermore, it is likely that in patients more than one mediator is active in inhibiting responses.5 Determining whether the effect of C5a-mediated dysfunction on the acquisition of nosocomial infection is independent of other demographic and clinical factors requires a larger cohort of patients and will be the focus of future work.
In conclusion, we have determined a mechanism by which C5a can inhibit phagocytosis and have demonstrated that neutrophils from critically ill patients show similar mechanistic responses when presented with the same target. Furthermore, we have demonstrated the utility of neutrophil surface CD88 as a biomarker for dysfunction and increased risk of nosocomial infection.
The online version of this article contains a data supplement.
Presented at the European Intensive Care Society Annual Congress, Barcelona, Spain, October 10-11, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the help and support of the medical and nursing staff of Ward 118 (General Intensive Care Unit) of the Royal Infirmary of Edinburgh. We are also grateful to Ms Kirsty Everingham and Mr David Hope (research coordinators in the Department of Anesthesia, Critical Care and Pain Medicine, University of Edinburgh), and to the Edinburgh Critical Care Research Group. We are also grateful to Mrs Shonna Johnston and Mrs Fiona Rossi, Center for Inflammation Research, University of Edinburgh, for their help and assistance with flow cytometry.
This study was funded by the Chief Scientist Office, NHS Scotland (grant CAF/08/13), the Sir Jules Thorn Charitable Trust, and NHS Lothian Research and Development fund. D.J.D. is a Wellcome Trust–funded senior research fellow.
Wellcome Trust
Authorship
Contribution: A.C.M. designed, obtained funding for, and performed the research and analysis, and wrote the manuscript; M.B., L.C.B., N.A.M., K.D., R.O.J., and A.M. performed the research and revised the manuscript; T. S. Wilkinson conceived the project, obtained funding, and revised the manuscript; D.F.M. modified the design of the study and revised the manuscript; J.A. and C.M. recruited patients and collected data; C.H. obtained the funding and revised the manuscript; A.W.H., D.G.S., and I.F.L. designed the infection definitions, participated as expert adjudication panel members, and revised the manuscript; N.A. designed the study, obtained funding, analyzed the data, and revised the manuscript; D.J.D. designed the study and revised the manuscript; A.G.R. designed the study, advised on technical aspects of experimental methods, and revised the manuscript; T. S. Walsh designed the study, obtained funding, supervised the project, designed the infection definitions, participated as an expert adjudication panel member, and revised the manuscript; A.J.S. designed the study, obtained funding, designed the infection definitions, participated as an expert adjudication panel member, supervised the project, and wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: A. Conway Morris, Rm C2.17, MRC/University Centre for Inflammation Research, Queen's Medical Research Institute, 47 Little France Crescent, Edinburgh, United Kingdom, EH16 4TJ; e-mail: mozza@doctors.org.uk.