Abstract
MHC class I (MHC I) is essential to NK- and T-cell effector and surveillance functions. However, it is unknown whether MHC I polymorphism influences adaptive immunity through NK cells. Previously, we found that MHC I Dk, a cognate ligand for the Ly49G2 inhibitory receptor, was essential to NK control of murine (M)CMV infection. Here we assessed the significance of NK inhibitory receptor recognition of MCMV on CD8 T cells in genetically defined MHC I Dk disparate mice. We observed that Dk-licensed Ly49G2+ NK cells stabilized and then enhanced conventional dendritic cells (cDCs) recovery after infection. Furthermore, licensed NK support of cDC recovery was essential to enhance the tempo, magnitude, and effector activity of virus-specific CD8 T cells. Minimal cDC and CD8 T-cell number differences after low-dose MCMV in Dk disparate animals further implied that licensed NK recognition of MCMV imparted qualitative cDC changes to enhance CD8 T-cell priming.
Introduction
Natural killer (NK) cells are essential mediators of virus immunity1 ; their deficiency in humans or depletion from mice leads to uncontrolled viral replication and poor clinical outcome.2-4 MHC class I (MHC I) molecules, which are ligands for polymorphic human KIR and mouse Ly49 receptors, play a critical role in NK-cell activation and self-tolerance. The absence of self-MHC I renders cells susceptible to NK-cell cytotoxicity.5-7 Moreover, the interaction between MHC I and NK-cell inhibitory receptors is critical in NK-cell acquisition of self-tolerance and functional competence.8-11 Indeed, NK inhibitory receptor recognition of virus-infected cells displaying reduced or altered self-MHC I is a paradigm for the field. Despite this, only activation receptors have so far been shown to confer specific recognition and control of virus infection by NK cells; the significance of NK inhibitory receptors in virus recognition therefore is debated.12
In MA/My mice and other H-2k strains, the MHC provides vital protection against lethal murine CMV (MCMV) infection.13-15 Classic genetics studies to examine MA/My virus resistance mapped genes to the MHC I D region on chromosome 17 and the NK gene complex (NKC) on chromosome 6.15-18 Recently, we identified MHC I Dk as a critical genetic factor,19 and further demonstrated a requisite role of licensed Ly49G2+ NK cells needed in MCMV resistance.18,19 Consistent with a requirement for NK stimulatory signals, Ly49P has been shown to bind MHC I Dk on infected cells displaying MCMV glycoprotein gp34.20 Thus, virus sensing via Ly49G2 and Ly49P receptors likely combine to drive potent antiviral NK responses after MCMV infection in H-2k mice. The intriguing finding of Babic et al recently demonstrating how MCMV gp34 associated with MHC I on infected cells may enhance interaction with NK inhibitory receptors and immune evasion strategies, further highlights that NK missing-self MHC I surveillance mechanisms contribute to virus control in vivo.21
As major effectors in front line antiviral defenses, NK cells further impact adaptive immunity through interactions with dendritic cells (DCs). NK-DC “crosstalk” can enhance NK cytotoxicity and proliferation, sustain splenic DC subsets after virus infection,22,23 and stimulate DC maturation and Ag-presenting functions.22-30 NK cells may thus provide needed signals during NK-DC crosstalk that promote tumor- or virus-specific CD8 T-cell immunity.23,31 In accord with this suggestion, high-affinity Ly49H recognition of MCMV m157 displayed on infected cells caused NK cells to affect the regulation of type I IFNs, DC subset retention and induction of virus-specific CD8 T-cell effectors.22,23,26 However, rapid containment of MCMV and Ag availability via Ly49H-mediated NK responses may also inadvertently contribute to viral persistence by reducing initial CD4 and CD8 T-cell responses.32 Whether NK inhibitory receptor recognition of virus infection affects dendritic cells and/or adaptive T-cell immunity has not been investigated. Such NK-cell recognition strategies may be crucial after infection with viruses known to manipulate MHC I expression.
To test our hypothesis and the in vivo effect of NK inhibitory receptor recognition of MCMV, we used immunogenetic strategies to examine DCs and virus-specific CD8 T cells in MHC I Dk disparate congenic and transgenic animals. We show that splenic DCs were at first diminished by 2 days after viral infection, irrespective of NK cell–mediated virus control. This was perpetuated in mice lacking NK inhibitory receptor recognition of MCMV. In contrast, mice displaying NK inhibitory receptor MCMV recognition retained and recovered splenic DCs and significantly enhanced CD8 T-cell immunity. Our findings implicate an indirect, yet profound role for NK cells wielding inhibitory receptor (ie, missing-self) recognition of target cells to stimulate antigen-specific CD8 T-cell effector responses and further demonstrate how MHC I polymorphism can dramatically shape adaptive viral immunity through its effect on innate NK cells.
Methods
Mice and genotyping
MA/My, C57L, and C57BL/6J mice (The Jackson Laboratory) and MHC I Dk congenic C57L.M-H2k(R7), C57L.M-H2k(R2), and transgenic (Tg3-Dk) mice generated previously18,19 were maintained under specific pathogen-free conditions at the University of Virginia (UVA), which is AAALAC accredited, and managed with The Jackson Laboratory's Colony Management System (Version 4.1.2). All animal studies were approved by and conducted in accordance with the UVA Animal Care and Use Committee oversight. Congenic and transgenic mice were genotyped as described previously using MHC genetic markers 17Mit16, 17Uva12, 17Uva17, 17Uva03, and H-2D.18,19
Virus studies and in vivo resistance assays
Salivary gland stock virus (SGV) was prepared and titered on NIH3T3 monolayers as described.15 Experimental mice (8-12 weeks) were infected intraperitoneally with 104 PFU MCMV, unless indicated otherwise. MCMV genomes in spleen samples were measured using quantitative real-time PCR as described.15 Minitab (Version 15; Minitab Inc) was used to generate box-and-whisker plots depicting interquartile ranges and whisker extensions to extreme values.
For in vivo NK-cell studies, NK cells or the Ly49G2 subset were depleted via PK136 or 4D11 mAb treatment, respectively, and immunodepletions were confirmed as described.18 CD4 T-cell depletions were performed as described.18 To examine a CD8 T-cell response to the Db-restricted epitope HGIRNASFI (Genscript), mice were injected with 500 μg of peptide, and 50 μg each of polyI:C (InvivoGen) and agonist αCD40 Ab (FGK45.5), as described.33 Control mice were injected with SSPPMFRV (Genscript) peptide.
Abs and flow cytometry
Anti–mouse NKp46/NCR1 was purchased from R&D Systems. Anti–mouse CD4 (L3T4), CD11c (N418), Ly49C/I/F/H (14B11), CD49b (DX5), NK1.1 (PK136), CD11b (M1/70), CD90.2 (Thy1.2; 53-2.1), TNF-α (MP6-XT22), and streptavidin-allophycocyanin-Cy7 were purchased from eBioscience. Anti–mouse IFN-γ (XMG1.2), CD4 (GK1.5), CD8 (53-6.7), Ly49G2 (4D11), CD3 (145-2C11), I-A/I-E MHC II (2G9) were purchased from BD PharMingen. Anti–mouse PDCA-1(927), CD3 (145-2C11), and CD19 (6D5) were purchased from Biolegend. M45-HGIRNASFI-Db and M139-TVYGFCLL-Kb tetramers were kindly provided by the NIH Tetramer Core Facility. Live/dead discrimination was performed using LIVE/DEAD Fixable violet according to the manufacturer's protocol (Invitrogen). To set up compensation for the fixable violet dye and tandem dyes for antibodies that stain rare spleen populations, the ArC Amine Reactive Compensation Bead Kit (Invitrogen) and the AbC anti-Rat/Hamster Bead Kit (Invitrogen) were used, respectively. Purified PK136, 24G2, GK1.5, and 4D11 mAbs were obtained from hybridoma supernatants at the UVA Lymphocyte Culture Center (Charlottesville, VA). Rat polyclonal IgG was purchased from Sigma-Aldrich. Functional-grade rat IgG2a was purchased from eBioscience.
Ex vivo dendritic cells were prepared from spleens as described.34 For analysis of the MCMV-specific CD8 T response over time, mice were bled at indicated times after infection and lymphocytes prepared from blood. All Ab stains were performed on ice. Ab-labeled cells were analyzed by flow cytometry on a FACScan, FACSCanto I, or FACSCanto II (BD Biosciences). Data were collected using FACSDiva software (BD Biosciences) and analyzed using FlowJo (Versions 8.0 and 9.2; Tree Star).
NK cytokine assay
Stimulation of splenocytes was performed as described.19 Briefly, splenocytes (8 × 105/well) resuspended in R10 complete media plus IL-2 (100 U/mL) and Brefeldin A (5 μg/mL) were incubated on immobilized mAb 24G2 (32 μg/mL) for 4 hours at 37°C. Control splenocytes were incubated with PMA (100 ng/mL; Sigma-Aldrich) plus ionomycin (0.7 μg/mL; Sigma-Aldrich) for 4 hours at 37°C. After stimulation, cells were stained for surface antigens and permeabilized (BD Cytofix/Cytoperm;BD Biosciences) to detect intracellular IFN-γ.
In vivo CTL assay
MCMV-specific CD8 T-cell cytotoxicity was determined as described.35 H-2b haplotype splenocytes from naive C57BL/6 mice pulsed with M45 HIGRNASFI or M139 TVYGFCLL viral peptides (Genscript; 1 μg/mL) were labeled with 0.3μM CFSE (Invitrogen); control unpulsed splenocytes were labeled with 3μM CFSE. CFSE-labeled targets (1.6 × 107 cells) resuspended in PBS were intravenously injected into test mice. Recipient splenocytes were harvested after 4 hours and analyzed in flow cytometry as described. Specific lysis was calculated as follows: 1 − ([average CFSEhi unpulsed targets/average CFSElo peptide-pulsed targets in uninfected mouse]/[average CFSEhi unpulsed targets/average CFSElo peptide-pulsed targets in infected mouse]) × 100%.
CD8 T-cell restimulation assay
On day 6 after MCMV infection, splenocytes resuspended in IMEM (Invitrogen) supplemented with 10% FBS, 10 U/mL penicillin G, 10 μg/mL streptomycin sulfate, 2mM l-glutamine, and 0.05% 2-ME were incubated for 5 hours ± the indicated peptides (1 μg/mL) plus Golgi-Stop (BD Biosciences; 1 μL/mL). For intracellular cytokine stains, cells were stained for surface Ags before permeabilization using a kit (BD Cytofix/Cytoperm; BD Biosciences) and further staining for intracellular IFN-γ and TNF-α.
Statistical analysis
Data are presented as means ± SD. Minitab (Version 15; Minitab Inc) was used to compare samples by Student t test, unless otherwise indicated.
Results
NK cells with inhibitory Ly49G receptor recognition of MCMV promote recovery of cDCs after viral infection
Recent studies have shown that early NK-cell responses and MCMV resistance because of Ly49H activation receptor recognition of infected cells is linked with retention of spleen DCs and induction of effector CD8+ T cells after viral infection.23,26,36 Together, NK cells and CD8+ T cells supply essential control of viral loads.37,38 Because CD8α+ DCs are needed to establish CD8+ T-cell immunity after viral infection,39 an indirect, yet helpful, result of NK protection of DCs is to promote CD8+ T-cell immunity. Despite recent advances toward clarifying how NK responses enhance adaptive immune effector mechanisms, it is unknown whether inhibitory receptor-mediated NK-cell virus recognition can influence virus-specific CD8 T cells.
To address this question, we analyzed the effect of MCMV on spleen DCs and CD8+ T cells in MHC I Dk disparate mice with or without NK-cell inhibitory Ly49G receptor-mediated virus recognition and resistance.19 MHC I Dk is a known cognate ligand of Ly49G40,41 which licenses Ly49G2+ NK cells in Dk transgenic and congenic strains (Xie et al19 and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). As shown in Figure 1A, congenic R7 (Dk) mice efficiently controlled MCMV, whereas virus loads were approximately 1000-fold higher in R2 (no Dk) spleen on day 3.5 and were still measurable on day 12. To assess DC subsets, we stained splenocytes for CD3, CD19, CD11c, and MHC II. Viable CD3−CD19−CD11chi MHC II+ DCs were comparable in R7 and R2 before infection (Figure 1B-C). Two days later, CD11chi cDCs decreased significantly in both strains. This was followed by cDC recovery in R7 on day 3.5; however, both the proportion and numbers of cDCs remained significantly decreased in R2 (Figure 1B-C). Although total R2 splenocytes were approximately 1.3-fold decreased, such a minor difference was unlikely to explain a greater than 5-fold decline in R2 cDC (Figure 1C-D). These data demonstrate that splenic cDCs recovered exclusively in MHC I Dk mice. That cDC numbers were responsive to virus control in R7 mice suggested that NK-cell responses might enhance cDC recovery. To address this, we assessed the influence of NK cells on splenic cDCs after infection. We treated R7-NKCmamy mice with mAb to eliminate total (NK1.1/PK136) or just Ly49G2 (4D11) NK cells before infection. As shown in Figure 1E, cDCs were significantly decreased in infected mice after either treatment. These data demonstrate that NK cells aided cDC recovery in infected MHC I Dk mice; without licensed Ly49G2+ NK cells, an upturn in cDC was not observed.
Ly49G+ NK cells in MHC I Dk mice promote recovery of cDCs after MCMV infection. (A) Median MCMV genome values for spleens of R7 and R2 congenic mice at the indicated times after infection. (B-D) Control and MCMV-infected R7 and R2 splenocytes were stained for CD3, CD19, MHC class II, and CD11c and analyzed by flow cytometry. (B) Representative dot plots showing the proportion of CD11chi, MHC II+ cDCs in uninfected and infected R7 and R2 mice. The dot plots were gated on live CD3−, CD19− cells (C) The graph represents the mean ± SEM for live gated cDCs in R7 and R2. (D) The graph represents the mean ± SEM for total splenocytes in R7 and R2. (A-D) Data are representative of 5 experiments with 3-4 mice per strain per time point (*P < .01, **P < .005, ***P < .0005 strain comparison at the indicated time point by Student t test). (E) The graph represents the mean ± SEM for live gated cDCs in R7, NK cell–depleted R7 (PK136) and Ly49G2+ NK cell–depleted (4D11) mice on day 3.5 after infection. Data are representative of 2 experiments with 4 to 6 mice per treatment group (*P < .01, **P < .005 by Student t test compared with rat IgG treated, no significant difference between uninfected and rIgG treated).
Ly49G+ NK cells in MHC I Dk mice promote recovery of cDCs after MCMV infection. (A) Median MCMV genome values for spleens of R7 and R2 congenic mice at the indicated times after infection. (B-D) Control and MCMV-infected R7 and R2 splenocytes were stained for CD3, CD19, MHC class II, and CD11c and analyzed by flow cytometry. (B) Representative dot plots showing the proportion of CD11chi, MHC II+ cDCs in uninfected and infected R7 and R2 mice. The dot plots were gated on live CD3−, CD19− cells (C) The graph represents the mean ± SEM for live gated cDCs in R7 and R2. (D) The graph represents the mean ± SEM for total splenocytes in R7 and R2. (A-D) Data are representative of 5 experiments with 3-4 mice per strain per time point (*P < .01, **P < .005, ***P < .0005 strain comparison at the indicated time point by Student t test). (E) The graph represents the mean ± SEM for live gated cDCs in R7, NK cell–depleted R7 (PK136) and Ly49G2+ NK cell–depleted (4D11) mice on day 3.5 after infection. Data are representative of 2 experiments with 4 to 6 mice per treatment group (*P < .01, **P < .005 by Student t test compared with rat IgG treated, no significant difference between uninfected and rIgG treated).
Splenic cDCs were further examined in transgenic (Tg-Dk) mice to assess whether Dk is crucial to recovery. As expected, a greater proportion and increased numbers of cDCs were observed on day 3.5 in Tg-Dk compared with nontransgenic (non-Tg) mice (Figure 2A), albeit Tg-Dk splenic cDC recovery was less dramatic than in R7 (compare Figures 1,2). This result demonstrates that MHC I Dk, and possibly an unknown gene(s) product linked to Dk in R7, but missing in C57L, R2 and Tg-Dk mice, regulates cDC recovery. A recovery difference was further reflected in CD11b+ and CD8α+ DC subset differences in Tg-Dk and non-Tg mice (Figure 2B-D) and in congenic R7 and R2 mice (supplemental Figure 2) after MCMV infection. However, we have not observed differences in pDCs (CD3−, CD19−, MHC II+, CD11cmid, PDCA-1+) in the strains before or after infection (supplemental Figure 2). Together with Figure 1, these data demonstrate that MHC I Dk virus resistance through Ly49G2+ NK cells is needed to recover splenic cDCs during acute MCMV infection.
MHC I Dk is sufficient to support splenic DCs after MCMV infection. (A) Representative dot plots showing the proportion of CD11chi, MHC II+ cDCs in uninfected and infected Tg-Dk and non-Tg spleens. The dot plots were gated as described for Figure 1. (B) Representative dot plots showing the proportion of CD8α+ and CD11b+ cDC subsets in uninfected and infected Tg-Dk and non-Tg spleens. The dot plots were gated on CD3−, CD19−, CD11chi, MHC II+ cells. (C) The graph represents the mean ± SEM for live gated cDC subsets in Tg-Dk spleen. (D) The graph represents the mean ± SEM for live gated cDC subsets in non-Tg spleen. The data are representative of 4 experiments with 4 mice per strain per time point. Both CD8α+ and CD11b+ cDC subsets were significantly higher on day 3.5 in Tg-Dk than non-Tg mice (P < .001), whereas cDC subsets were comparable in uninfected animals (*P < .05, **P < .001 compared with respective subset in uninfected mice by Student t test).
MHC I Dk is sufficient to support splenic DCs after MCMV infection. (A) Representative dot plots showing the proportion of CD11chi, MHC II+ cDCs in uninfected and infected Tg-Dk and non-Tg spleens. The dot plots were gated as described for Figure 1. (B) Representative dot plots showing the proportion of CD8α+ and CD11b+ cDC subsets in uninfected and infected Tg-Dk and non-Tg spleens. The dot plots were gated on CD3−, CD19−, CD11chi, MHC II+ cells. (C) The graph represents the mean ± SEM for live gated cDC subsets in Tg-Dk spleen. (D) The graph represents the mean ± SEM for live gated cDC subsets in non-Tg spleen. The data are representative of 4 experiments with 4 mice per strain per time point. Both CD8α+ and CD11b+ cDC subsets were significantly higher on day 3.5 in Tg-Dk than non-Tg mice (P < .001), whereas cDC subsets were comparable in uninfected animals (*P < .05, **P < .001 compared with respective subset in uninfected mice by Student t test).
Licensed NK cells enhance T-cell induction and expansion after viral infection
That Ly49G2+ NK cells protected critical cDCs after MCMV infection suggested that efficient inhibitory receptor recognition of viral infection might affect CD8 T-cell immunity. To address this question, we examined splenic CD8 T cells in Dk disparate mice after MCMV infection. Virus-specific T cells were visualized after staining with Db and Kb tetramers loaded with M45 and M139 viral peptides, respectively. Importantly, both reagents stain T cells from R2 and R7 mice made H-2 heterozygous (ie R2het and R7het) via backcrossing to C57L. As shown in Figure 3A, CD8 T cells were approximately 4-fold more abundant by day 6 in R7 mice. Before day 4, T cells had not expanded (data not shown) and did not contribute significant virus control.18 In accord with this, tetramer-positive CD8 T cells were evident in R7 on day 6 (Figure 3B). In contrast, approximately 40-fold fewer virus-specific T cells were observed in R2 (Figure 3C). Thus, MHC I Dk led to vastly different CD8+ T-cell responses in R7 and R2 after MCMV infection.
Effect of MHC I Dk virus resistance on MCMV-specific CD8 T-cell response. Control and MCMV-infected R7 and R2 splenocytes were stained for CD19, CD3, CD8, and virus-specific T-cell receptors using M45-Db tetramers before flow cytometric analysis. (A) The graph represents the mean ± SEM for live gated CD8+ T cells (CD19−, CD3+) in uninfected and infected spleens from R7 and R2 mice. (B) Representative dot plots showing the proportion of M45-Db–specific CD8 T cells in uninfected and infected R7 and R2 spleens. The dot plots were gated on CD19−, CD3+ cells. (C) The graph represents the mean ± SEM for virus-specific CD8 T cells in uninfected and infected R7 and R2 spleens. (D) The graph represents the mean ± SEM for virus-specific CD8 T cells in blood collected from serially bled R7 animals. (A-C) The data are representative of 4 experiments with 4 mice per strain per time point. (D) The data are representative of 2 experiments with 4 mice per strain per time point (*P < .005, **P < .001, ***P < .0005, by Student t test).
Effect of MHC I Dk virus resistance on MCMV-specific CD8 T-cell response. Control and MCMV-infected R7 and R2 splenocytes were stained for CD19, CD3, CD8, and virus-specific T-cell receptors using M45-Db tetramers before flow cytometric analysis. (A) The graph represents the mean ± SEM for live gated CD8+ T cells (CD19−, CD3+) in uninfected and infected spleens from R7 and R2 mice. (B) Representative dot plots showing the proportion of M45-Db–specific CD8 T cells in uninfected and infected R7 and R2 spleens. The dot plots were gated on CD19−, CD3+ cells. (C) The graph represents the mean ± SEM for virus-specific CD8 T cells in uninfected and infected R7 and R2 spleens. (D) The graph represents the mean ± SEM for virus-specific CD8 T cells in blood collected from serially bled R7 animals. (A-C) The data are representative of 4 experiments with 4 mice per strain per time point. (D) The data are representative of 2 experiments with 4 mice per strain per time point (*P < .005, **P < .001, ***P < .0005, by Student t test).
Interestingly, recent data support that NK Ly49H activation receptor recognition of MCMV can affect virus-specific CD8 T-cell immunity by altering the kinetics and cytotoxicity of the CD8+ T-cell response in vivo. Comparing Ly49H disparate mice, Robbins et al found that virus-specific CD8 T cells with cytotoxic effector function accumulated by day 4 in Ly49H mice.23 Although delayed, virus-specific CTLs with comparable functional activity eventually developed 5 days postinfection in mice without Ly49H.23 In a related study, Andrews et al found fewer MCMV-specific CD8 T cells at day 6 in Ly49H mice.32 Yet, no difference in CTL activity was observed until it declined sharply in Ly49H mice 8 days after infection.32 Relative variations in the tempo or function of CD8 T cells in the studies could be related to virus strain or in vivo cytotoxicity assay differences. Nonetheless, the recent findings together supports that NK activation receptor recognition of MCMV via Ly49H significantly affected the timing of T-cell effector acquisition during viral infection.23,32
We questioned whether inhibitory receptor recognition of viral infection could have a similar impact on the tempo and magnitude of CD8 T-cell immunity after MCMV infection. To address this, we monitored T-cell responses over time in the blood of R2 and R7 mice. As with results in spleen, M45-Db-specific CD8 T cells were far more abundant in R7 blood at day 6 (Figure 3D). By day 12, R7 virus-specific T cells had contracted substantially. In apparent contrast to results in R7 or Ly49H disparate mice, R2 MCMV-specific CD8 T cells never achieved a response level comparable with that in R7 at day 6 (Figure 3D). We considered that R2 might be incompetent to generate M45-Db–specific T cells. To test this in a separate experiment, we immunized R2 mice with M45 or control M38 peptides plus adjuvant. Six days later, M45-specific CD8 T cells were readily visualized among ex vivo splenocytes after tetramer staining (supplemental Figure 3). This result demonstrated that M45-Db–specific T-cell immunity was intact in R2; however, the absence of MHC I Dk resulted in poor CD8+ T-cell stimulation during MCMV infection. Thus, MHC polymorphism, manifest through NK-cell responsiveness, exerted a fundamental shift in CD8 T-cell immunity.
To account for observed differential CD8 T-cell responses, we considered that CD4 T cells in R7 could have enhanced viral immunity, as their contribution to MCMV resistance in salivary glands and lungs has been reported.42,43 Recent studies further documented CD4 T-cell expansion and activation after MCMV infection, their capacity to shape CD8+ T-cell memory, and a role in persistent MCMV infection in mice without Ly49H.32,44,45 To test this, we examined virus-specific CD8+ T cells in CD4 T cell–depleted R7 mice (supplemental Figure 4). In accord with previous studies, viral loads were comparable in salivary gland, liver, and spleen shortly after infection (data not shown). Likewise, the frequency (supplemental Figure 4A) and number of total (supplemental Figure 4B) and MCMV-specific CD8+ T cells were comparable in control and CD4 T cell–deficient mice. These data therefore demonstrated that enhanced MCMV-specific CD8 T-cell responsiveness in R7 is CD4 T-cell-independent.
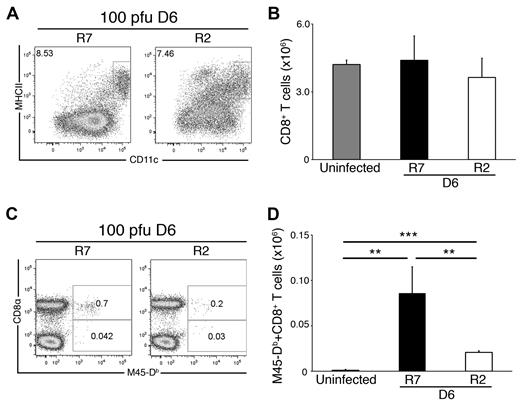
We next considered that inept CD8+ T-cell induction in R2 might be attributed to a difference in cDC numbers after infection. To address this question, we examined cDC and CD8 T cells in spleen after a low-dose (100 PFU) MCMV infection regimen so as to minimize host cell perturbations. Following this protocol, we observed that the frequency of cDCs was comparable in the 2 strains and MCMV levels were at or below detection level (Figure 4A and data not shown). Likewise, total CD8+ T-cell counts were comparable in both strains (Figure 4B). Despite these similarities, R7 had 4 times as many M45-Db–specific CD8 T cells as R2 on day 6 (Figure 4C-D). Furthermore, there was no difference in the absolute numbers of virus-specific CD8 T cells after low or higher dose infection in R2. These data demonstrate that the effect of MHC I Dk on NK-cell resistance to virus was not merely quantitative and further suggest that a qualitative difference in splenic cDCs may lead to altered CD8 T-cell immune responses.
CD8 T-cell priming defect in susceptible mice without MHC I Dk. Ex vivo splenocytes isolated from MCMV-infected (100 PFU) R2 and R7 mice were stained and analyzed for cDCs as described in Figure 1. Data are representative of 2 experiments with 3-4 mice per genotype. (A) Representative dot plots showing the proportion of CD11chi, MHC II+ cDCs in day 6–infected R2 and R7 spleens. The dot plots were gated as described for Figure 1. (B) The graph represents the mean ± SEM for live gated CD8+ T cells (CD19−, CD3+) in uninfected and day 6–infected R7 and R2 spleens. (C) Representative dot plots showing the proportion of M45-Db–specific CD8 T cells in day 6–infected R7 and R2 spleens. The dot plots were gated on CD19−, CD3+ cells. (D) The graph represents the mean ± SEM for virus-specific CD8 T cells in uninfected and day 6–infected R7 and R2 spleens. The data are representative of 2 experiments with 4 mice per strain per time point (**P < .01, ***P < .0001 by Student t test).
CD8 T-cell priming defect in susceptible mice without MHC I Dk. Ex vivo splenocytes isolated from MCMV-infected (100 PFU) R2 and R7 mice were stained and analyzed for cDCs as described in Figure 1. Data are representative of 2 experiments with 3-4 mice per genotype. (A) Representative dot plots showing the proportion of CD11chi, MHC II+ cDCs in day 6–infected R2 and R7 spleens. The dot plots were gated as described for Figure 1. (B) The graph represents the mean ± SEM for live gated CD8+ T cells (CD19−, CD3+) in uninfected and day 6–infected R7 and R2 spleens. (C) Representative dot plots showing the proportion of M45-Db–specific CD8 T cells in day 6–infected R7 and R2 spleens. The dot plots were gated on CD19−, CD3+ cells. (D) The graph represents the mean ± SEM for virus-specific CD8 T cells in uninfected and day 6–infected R7 and R2 spleens. The data are representative of 2 experiments with 4 mice per strain per time point (**P < .01, ***P < .0001 by Student t test).
To ascertain NK-cell contributions to this difference, we measured CD8 T-cell responses after MCMV infection in NK-cell depleted mice. Comparable with results in R2, R7 mice depleted of total or Ly49G2+ NK cells had severely impaired M45- and M139-specific CD8+ T cells at day 6 (Figure 5). These results demonstrated that Ly49G2+ NK cells were essential to enhance CD8+ T cells in MHC I Dk mice. To assess whether Dk was required, CD8 T cells were further examined in Tg-Dk and non-Tg control mice after infection. As shown in Figure 6A, the frequency of M45-specific CD8 T cells was significantly enhanced in Tg-Dk animals. These data plainly establish a significant and substantive impact of MHC I polymorphism on CD8 T-cell virus immunity via self-MHC I Dk licensed Ly49G+ NK-cell recognition and control of viral infection.
Ly49G2+ NK cells key to priming MCMV-specific CD8 T-cell response. (A) Representative dot plots showing the proportion of M45-Db–specific CD8 T cells in spleens of uninfected R7, infected R7 treated with control rat IgG, infected R7 depleted of NK cells (PK136) and infected R7 depleted of Ly49G2+ NK cells (4D11). The dot plots were gated on CD19−, CD3+ cells. (B) The graph represents the mean ± SEM for live gated M45-Db–specific CD8 T cells in uninfected and infected R7 animals given the indicated treatments. (C) The graph represents the mean ± SEM for live gated M139-Kb–specific CD8 T cells in uninfected and infected R7 animals given the indicated treatments. The data are representative of 4 experiments with 3-4 mice per treatment group (**P < .005, ***P < .0005 by Student t test compared with rat IgG treated).
Ly49G2+ NK cells key to priming MCMV-specific CD8 T-cell response. (A) Representative dot plots showing the proportion of M45-Db–specific CD8 T cells in spleens of uninfected R7, infected R7 treated with control rat IgG, infected R7 depleted of NK cells (PK136) and infected R7 depleted of Ly49G2+ NK cells (4D11). The dot plots were gated on CD19−, CD3+ cells. (B) The graph represents the mean ± SEM for live gated M45-Db–specific CD8 T cells in uninfected and infected R7 animals given the indicated treatments. (C) The graph represents the mean ± SEM for live gated M139-Kb–specific CD8 T cells in uninfected and infected R7 animals given the indicated treatments. The data are representative of 4 experiments with 3-4 mice per treatment group (**P < .005, ***P < .0005 by Student t test compared with rat IgG treated).
MHC I Dk NK-cell virus resistance defines MCMV-specific CD8 T-cell effector response. Tg-Dk and non-Tg splenocytes were stained for flow cytometric analysis as described in Figure 3. (A) Representative dot plots showing the proportion of M45-Db–specific CD8 T cells in day 6–infected spleens. The dot plots were gated on CD19−, CD3+ cells. Data are representative of 4 experiments with 4 mice per strain per time point. (B) Representative dot plots showing the proportion of cytokine responsive CD8 T cells after peptide restimulation of splenocytes. The dot plots were gated on CD19−, CD3+, CD8+ cells. (C) The graph represents the mean ± SEM for cytokine producing M45-Db– and M139-Kb–specific CD8 T cells in uninfected and day 6–infected Tg-Dk and non-Tg spleens. Data are representative of 2 experiments with 4 mice per strain per time point (**P < .0005 by Student t test). (D) A 4-hour in vivo CTL assay was performed with M45 peptide-pulsed and unpulsed control targets. The graph represents the mean ± SEM for in vivo virus-specific CTL activity at the indicated times after infection. Virus-specific CTL activity in spleen was significantly enhanced in Tg-Dk compared with non-Tg on day 6 postinfection (P < .01). Data are representative of 2 experiments with 4 mice per strain per time point.
MHC I Dk NK-cell virus resistance defines MCMV-specific CD8 T-cell effector response. Tg-Dk and non-Tg splenocytes were stained for flow cytometric analysis as described in Figure 3. (A) Representative dot plots showing the proportion of M45-Db–specific CD8 T cells in day 6–infected spleens. The dot plots were gated on CD19−, CD3+ cells. Data are representative of 4 experiments with 4 mice per strain per time point. (B) Representative dot plots showing the proportion of cytokine responsive CD8 T cells after peptide restimulation of splenocytes. The dot plots were gated on CD19−, CD3+, CD8+ cells. (C) The graph represents the mean ± SEM for cytokine producing M45-Db– and M139-Kb–specific CD8 T cells in uninfected and day 6–infected Tg-Dk and non-Tg spleens. Data are representative of 2 experiments with 4 mice per strain per time point (**P < .0005 by Student t test). (D) A 4-hour in vivo CTL assay was performed with M45 peptide-pulsed and unpulsed control targets. The graph represents the mean ± SEM for in vivo virus-specific CTL activity at the indicated times after infection. Virus-specific CTL activity in spleen was significantly enhanced in Tg-Dk compared with non-Tg on day 6 postinfection (P < .01). Data are representative of 2 experiments with 4 mice per strain per time point.
NK cells direct the CD8 T-cell effector response
An impact of NK-cell resistance on antiviral CD8 T cells suggested that T-cell effector functions might differ as well. To examine this, we first used peptide-pulsed APCs in restimulation assays to measure T-cell effector cytokine responses. As shown in Figure 6B, virus-specific CD8 T cells in Tg-Dk and non-Tg mice were competent and produced significant IFN-γ on restimulation with M45 or M139 peptides. As expected, the number of Tg-Dk effector T cells significantly exceeded what was observed in non-Tg animals. Although we found that non-Tg effector T cells also more frequently produced TNF-α (Figure 6B), enumeration of multiple cytokine-producing virus-specific CD8 T cells revealed their greater abundance in Tg-Dk animals (Figure 6C). We next used peptide-pulsed and CFSE-labeled targets to assess virus-specific CD8 T-cell cytotoxicity in vivo. As shown in Figure 6D, M45-pulsed targets were efficiently eliminated from Tg-Dk spleen 6 days postinfection. Tg-Dk and non-Tg also displayed disparity in in vivo cytotoxicity against M139-pulsed targets (Table 1). The peak of in vivo CD8 T-cell killing occurred on day 8, followed by contraction of responsiveness in both strains on day 10. Notably, CTL activity decreased similarly in both strains so that non-Tg activity never exceeded that observed in Tg-Dk mice. These data demonstrate that although the magnitude of CD8 T-cell responding differs substantially, virus-specific and functionally competent effector T cells were still elicited in mice without highly effective NK inhibitory receptor recognition and control of virus infection. Thus, a major effect of NK inhibitory receptor recognition of MCMV-infected cells was to accelerate and significantly enhance effector CD8 T cells needed in viral immunity.
MHC I Dk NK cell virus resistance enhances MCMV-specific CTL activity
| Strain . | Average M139-specific killing, %* . |
|---|---|
| Tg | 68.9 ± 4.8 |
| Non-Tg | 45.1 ± 11.5 |
| Strain . | Average M139-specific killing, %* . |
|---|---|
| Tg | 68.9 ± 4.8 |
| Non-Tg | 45.1 ± 11.5 |
Tg indicates transgenic.
CTL activity was determined as described in Figure 6D. The difference in M139–specific killing was significant (P < .01).
Discussion
MHC I Dk is critical to MCMV resistance in MA/My and other H-2k strains of mice.19 More intriguing, NK cells marked by Ly49G2, a cognate inhibitory receptor for Dk, rapidly expand, undergo activation and deliver essential MCMV control after infection.18,19 Reduction of Dk surface expression in MCMV-infected cells should alert NK cells.15 Despite this, Babic et al recently showed that MCMV gp34 stabilizes MHC I binding to NK inhibitory receptors to promote immune evasion.21 Because Ly49P binds gp34-associated Dk on MCMV-infected cells,20 it may contribute to NK-mediated MCMV control. Nonetheless, without Ly49G2 receptors, Ly49p+ NK cells were unable to restrain MCMV synthesis after infection.19 Thus, Ly49G2 is required to improve NK control, presumably via recognition of MHC I Dk down-regulation on viral targets. An intriguing possibility under study, Ly49G2c57l receptor / ligand interactions may be hindered, and not stabilized, by MCMV gp34. Altogether, proficient missing-self Dk recognition by licensed Ly49G2+ NK cells significantly enhanced NK responses, likely in combination with Ly49P or another stimulatory receptor that can also respond to MCMV.19
On the basis of these observations, we tested the effect of MHC I polymorphism and Ly49G2+ NK-mediated MCMV resistance on adaptive virus immunity because the role of licensed NK inhibitory receptor recognition of virus-infected cells had not been examined before. We found unexpectedly that licensed NK-cell recognition of MCMV is paramount to recover splenic cDCs and to enhance virus-specific CD8 T cells after infection.
Previous reports established that splenic DCs are infected by and highly sensitive to MCMV; NK-cell Ly49H activation receptor recognition of MCMV m157 was shown to stabilize splenic cDCs after infection.22,23 Consistent with previous reports, Ly49G2+ NK-mediated MHC I Dk virus resistance was vital to splenic cDC subsets (Figures 1,2). Hence, NK support of splenic DCs may be because of their ability to limit infection in cDCs or their precursors, of which a small pool exists in the spleen itself.32,46 Because cDC subset half-lives are relatively short (1.5-2.9 days)47 and splenic cDC numbers drop significantly in resistant and susceptible mice after infection, NK inhibitory receptor-mediated virus resistance may curtail precursor infection so that cDC recovery occurs within several days. Still alternate possibilities advanced in recent work include NK restraint of infection in hematopoietic precursors of cDCs and/or NK regulation of high type I IFN levels and consequent negative effects, which could significantly hinder precursor DC recovery.23,27 The effect of licensed NK-cell virus resistance on splenic DCs and their precursors is therefore instructive.
Interestingly, Robbins et al further linked Ly49H-m157 MCMV recognition and cDC support to prompt induction and regulation of virus-specific CD8 T-cell effectors.23 This finding exemplifies the essential role of CD8α+ cDCs in priming CD8 T-cell immunity to viruses.39 Hence, a reduction in cDC numbers, and especially the CD8α+ subset (Figure 2, supplemental Figure 2) may partly account for disparity observed in CD8 T-cell priming in resistant and susceptible mice. Support of a qualitative distinction in cDCs needed to enhance T-cell immunity was also evident. First, after low-dose virus infection, cDC and CD8+ T-cell numbers in R2 and R7 were comparable, but virus-specific CD8 T cells were significantly expanded in R7 (Figure 4); second, after higher-dose MCMV infection, splenic cDC losses at 48 hours were a common feature shared by resistant and susceptible strains, yet cDC recovery and CD8 T-cell number differences still distinguished virus-specific T-cell immunity 6-18 days after infection in paired strains with disparate MHC I Dk directed NK resistance; and third, licensed NK cells directed a distinct virus-specific CD8 T cytokine response dominated by IFN-γ single producers. Although fewer in number, a much larger proportion of CD8 T effectors in non-Tg mice were IFN-γ+TNF-α+. We are currently investigating whether NK cells influence the acquisition of polyfunctional T cells described in humans and mice.48,49 Thus, self-MHC I Dk recognition via licensed Ly49G+ NK cells sustained splenic cDC numbers and further shaped their capacity to elicit and support adaptive virus immunity. Recently, it was suggested that efficient NK-mediated viral control in Ly49H mice may limit DC infection and consequently antigen availability to T cells.32 We observed herein that MHC I Dk MCMV control via Ly49G+ NK cells was essential to protect and recover cDCs, and to expand virus-specific effectors. These findings distinguish differential consequences associated with MCMV detection which relied more on NK activation or inhibitory receptor-mediated recognition strategies.
The mode of NK-cell virus detection may further dictate the priming of adaptive virus immunity. High-affinity Ly49H recognition of MCMV m157 sustained splenic cDCs and gave prompt induction of antiviral T-cell effectors,23 although CD8 T cells eventually achieved greater proliferation and effector activity in BALB/c mice without Ly49H+ NK control.32 In the current work, Ly49G2+ NK enhancement of CD8 T-cell numbers with full effector potential in Dk mice was remarkable. In contrast, CD8 T cells were acquired at later times after infection, the peak of CD8 T-cell numbers was substantially lower, and the cytotoxic capacity of CD8 T-cell effectors was at least significantly delayed in mice without Dk-licensed Ly49G2+ NK cells. Whether the in vivo cytotoxicity assay under the conditions used had the resolving power to discern further differences in effector function at the height of T-cell responsiveness in both strains is still unresolved. Nonetheless, CD8 T-cell numbers contracted in both strains after day 8, which paralleled a decline in effector activity revealed by the in vivo cytotoxicity experiments. This feature further highlights that Dk-licensed Ly49G2+ NK control differs from Ly49H+ NK viral control; consequently, subtle differences in NK receptor sensing of cell targets that relies more heavily on inhibitory or stimulatory receptors apparently has considerable influence over the timing, magnitude, and nature of adaptive T-cell responses.
Because Ly49G2 expression has been observed on activated CD8 T cells in LCMV infection,50 we considered that it could have directly influenced T-cell responses. However, we have not observed Ly49G2 expression on cDCs, CD8 T cells or virus-specific CD8 T cells after MCMV infection in the relevant strains (supplemental Figure 5). Although effective CTLs were eventually raised in either setting, CD8 T effector numbers were exceedingly low at all time points under study in mice without efficient recognition of viral infection because of altered MHC I Dk (Figures 3,6). Whether a compensatory mechanism leads to delayed viral clearance observed in these mice is unknown. Regardless, our findings establish a vital contribution of NK inhibitory receptors which survey for missing-self cues on infected target cells; recognition is a potent stimulus to activate and expand NK-cell antiviral responses, sustain cDCs, and then to enhance CD8 T-cell immunity.
An interesting hypothesis supported by the current findings, human NK-cell detection of virus infection via inhibitory receptors may coordinate CTL responses after infection with viral pathogens. Recent studies have implicated combined HLA and KIR genotypes with disease resistance effects among patients with chronic HIV or HCV infection.51,52 In particular, inhibitory KIR3DL1 and certain Bw4 alleles (B*57 and B*27) were associated with lower HIV viral load and slower disease progression.52 As Bw4 molecules are ligands of KIR3DL1,53 it has been proposed that a potent missing-self response on HIV modulation of HLA leads to virus control.52 Because Bw4 can license KIR3DL1 NK cells,10 the current model hints that delayed AIDS progression in KIR3DL1 and HLA-Bw4 individuals may be affected by NK inhibitory receptors coordinating adaptive virus immunity. As such, licensed NK-cell detection of viral targets could provide added support of productive NK-DC interactions, as observed in aviremic HIV patients.54 Moreover, licensed NK cells may further curtail DC infection and early viral dissemination before needed HIV-specific CD8 T-cell effectors undergo priming, expansion, and activation, which could be especially critical in chronic infections like HIV and HCV where certain cognate inhibitory NK receptor/HLA class I ligand pairings are associated with improved disease outcomes.
Licensed NK-cell virus control and its coordination of the adaptive immune response highlights a need to further examine the effect of MHC I polymorphism on MCMV resistance and viral immunity. Further study of self-MHC I–licensed NK-cell immunity will provide considerable insight into NK-cell biology, allow for the dissection of the molecular cues that link NK and CD8 T cells, and may provide new strategies to stimulate advantageous adaptive immune responses to both viruses and tumors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mariapia Degli-Esposti, Colin Brinkmann, Jie Sun, and members of the Brown Laboratory for valuable discussions. We thank Alyssa Lundgren and Jessica Prince for technical assistance.
This work was supported by National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases grants AI050072 and AI083024 (MGB). M.D.S. and E.R.C. were supported by the NIH Biotechnology Training Program (T32 GM08715) and the NIH Interdisciplinary Training Program in Immunology (5T32 AI07496), respectively.
National Institutes of Health
Authorship
Contribution: M.D.S., X.X., and E.R.C. designed and performed research; and M.D.S., T.N.B., and M.G.B. analyzed and interpreted data, designed experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for X.X. is Department of Molecular Physiology, Children's National Medical Center, 111 Michigan Ave NW, Washington, DC 20010.
Correspondence: Michael G. Brown, Department of Medicine, Box 801386, Beirne B. Carter Center for Immunology Research, University of Virginia Health System, 345 Crispell Dr, Charlottesville, VA 22908, e-mail: mgb4n@virginia.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal