Abstract
M-CSF favors the generation of folate receptor β–positive (FRβ+), IL-10–producing, immunosuppressive, M2-polarized macrophages [M2 (M-CSF)], whereas GM-CSF promotes a proinflammatory, M1-polarized phenotype [M1 (GM-CSF)]. In the present study, we found that activin A was preferentially released by M1 (GM-CSF) macrophages, impaired the acquisition of FRβ and other M2 (M-CSF)–specific markers, down-modulated the LPS-induced release of IL-10, and mediated the tumor cell growth–inhibitory activity of M1 (GM-CSF) macrophages, in which Smad2/3 is constitutively phosphorylated. The contribution of activin A to M1 (GM-CSF) macrophage polarization was evidenced by the capacity of a blocking anti–activin A antibody to reduce M1 (GM-CSF) polarization markers expression while enhancing FRβ and other M2 (M-CSF) markers mRNA levels. Moreover, an inhibitor of activin receptor-like kinase 4/5/7 (ALK4/5/7 or SB431542) promoted M2 (M-CSF) marker expression but limited the acquisition of M1 (GM-CSF) polarization markers, suggesting a role for Smad2/3 activation in macrophage polarization. In agreement with these results, expression of activin A and M2 (M-CSF)–specific markers was oppositely regulated by tumor ascites. Therefore, activin A contributes to the proinflammatory macrophage polarization triggered by GM-CSF and limits the acquisition of the anti-inflammatory phenotype in a Smad2-dependent manner. Our results demonstrate that activin A–initiated Smad signaling skews macrophage polarization toward the acquisition of a proinflammatory phenotype.
Introduction
Tissue-resident macrophages are phenotypically and functionally heterogeneous under homeostatic conditions because of their extreme sensitivity to the extracellular cytokine milieu.1 Although GM-CSF and M-CSF contribute to cell survival and proliferation, they exert distinct actions during macrophage differentiation. The lack of M-CSF alters the development of various macrophage populations,2 whereas GM-CSF–deficient mice only exhibit altered maturation of alveolar macrophages.3 Similarly, both cytokines promote the in vitro differentiation of macrophages with distinct morphology, pathogen susceptibility, and inflammatory function.4-7 GM-CSF gives rise to monocyte-derived macrophages with high antigen-presenting capacity that produce proinflammatory cytokines in response to TLR stimulation. Conversely, M-CSF leads to the generation of macrophages with high phagocytic activity and IL-10–producing ability in response to pathogens.7,8 Based on their respective cytokine profiles, human macrophages generated in the presence of GM-CSF or M-CSF are representative of the classic (M1) or alternative (M2) macrophage polarization states, respectively,7,9 and are considered proinflammatory and anti-inflammatory macrophages, respectively.7,9
Activins are pluripotent growth and differentiation factors of the TGFβ superfamily that are structurally composed of 2 β subunits (activin A, βAβA; activin AB, βAβB; activin B, βBβB) linked by a single covalent disulfide bond.10,11 The expression of activins is high in inflammatory pathologies, including rheumatoid arthritis and inflammatory bowel disease.12 Initially characterized as inducers of follicle-stimulating hormone production, activins are now known to regulate the growth of numerous cell types, to contribute to the maintenance of pluripotency of embryonic stem cells, and to exert antitumorigenic effects.13 Activins share their intracellular signaling pathway via Smad2/3 with other members of the TGFβ superfamily.13 Like TGFβ, activins regulate inflammatory responses and exert immunostimulatory and immunosuppressive functions at the T-cell level.13 Within the myeloid lineage, activin A has a central role in innate immunity and is considered to be a crucial modulator of inflammatory responses by virtue of its proinflammatory and regulatory activities.12 In fact, activin A modulates cytokine and chemokine release from myeloid cells14-16 and contributes to Langerhans cell differentiation both in vitro and in vivo.17 Activin A expression is up-regulated on activation and in response to inflammatory mediators,12,15,18 but is suppressed by glucocorticoids.19,20 The ability of activin A to induce arginase-1 expression in murine peritoneal macrophages has led to the proposal that activin A is a Th2 cytokine that promotes macrophage polarization toward the alternative phenotype.21
We previously analyzed the differences in gene expression between GM-CSF–polarized macrophages (M1 [GM-CSF] macrophages, hereafter referred to as M1 macrophages) and M-CSF–polarized macrophages (M2 [M-CSF] macrophages, hereafter referred to as M2 macrophages), and described the preferential expression of folate receptor β (FRβ) and heme-oxygenase 1 in in vitro–derived M2 macrophages and ex vivo tumor-associated macrophages (TAMs).22,23 To identify factors mediating the acquisition of their corresponding profiles, we investigated whether M1 (GM-CSF)–derived molecules influence the acquisition of M2 (M-CSF)–specific markers. We found that M1 macrophages secreted large amounts of functional activin A that promotes the expression of M1 (GM-CSF) markers, impairs the acquisition of M2 (M-CSF) markers, and down-regulates the production of IL-10. These results indicate that activin A contributes to macrophage polarization and shapes the inflammatory behavior of macrophages. Moreover, given the ability of macrophages to repolarize under the appropriate cytokine conditions,24 activin A might function, in an autocrine or paracrine manner, in regulating macrophage switch between polarization states.
Methods
Macrophage differentiation, cell culture, and flow cytometry
Human PBMCs were isolated from buffy coats of normal donors over a Lymphoprep (Nycomed Pharma) gradient according to standard procedures. Monocytes were purified from PBMCs by magnetic cell sorting using CD14 Microbeads (Miltenyi Biotec). Monocytes (> 95% CD14+ cells) were cultured at 0.5 × 106 cells/mL for 7 days in RPMI medium supplemented with 10% FCS (completed medium) at 37°C in a humidified atmosphere with 5% CO2 and containing 1000 U/mL of GM-CSF or M-CSF (10 ng/mL; ImmunoTools) to generate M1 and M2 monocyte–derived macrophages, respectively. Cytokines were added every 2 days. To generate monocyte-derived dendritic cells, monocytes were cultured at 0.7 × 106 cell/mL in complete medium containing GM-CSF (1000 U/mL) and IL-4 (1000 U/mL; ImmunoTools) for 5-7 days, with cytokine addition every second day. M1 macrophages generated in the presence of 1000 U/mL of GM-CSF were phenotypically identical to those generated in the presence of 50 or 200 U/mL of GM-CSF. However, they were distinct from monocyte-derived dendritic cells because they were devoid of cell-surface CD1a and CD209, retained CD14, and displayed lower levels of MHC class II (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Recombinant human activin A (2.5-25 ng/mL; Miltenyi Biotec) was added together with the indicated cytokine. To determine their effects on macrophage polarization, a blocking anti–activin antibody (100 ng/mL; R&D Systems) or an inhibitor of ALK4, ALK5, and ALK7 (SB431542, 10μM) was added every 24 hours. GM-CSF–free conditioned medium from M1 macrophages (at day 5) was generated by extensive washing of the cells 3 hours after GM-CSF addition and further culture for 48 hours. Murine M1 and M2 bone marrow–derived macrophages (BMDMs) were generated essentially as described previously9,25 using human M-CSF (10 ng/mL; ImmunoTools) or murine GM-CSF (20 ng/mL; Sigma-Aldrich), respectively. The mink lung epithelial cell line Mv1Lu26 was maintained in DMEM supplemented with 10% FCS. Phenotypic analysis was carried out by flow cytometry as described previously22 using either rabbit polyclonal antisera anti–human FRβ22,27 or rabbit preimmune serum,28 followed by FITC-labeled Fab goat anti–rabbit IgG. All incubations were done in the presence of 50 μg/mL of human IgG to prevent binding through the Fc portion of the antibodies.
Western blot analysis
Cell lysates were obtained in 10mM Tris-HCl pH 8, 150mM NaCl, 1% NP-40 lysis buffer containing 2mM Pefabloc, 2 μg/mL of aprotinin/antipain/leupeptin/pepstatin, 10mM NaF, and 1mM Na3VO4. Ten micrograms of cell lysates was subjected to SDS-PAGE and transferred onto an Immobilon polyvinylidene difluoride membrane (Millipore). After blocking of the unoccupied sites with 5% bovine serum albumin in a buffer containing 50mM Tris-HCl, pH 7.6, 150mM NaCl, and 0.1% Tween-20, protein detection was carried out using polyclonal antisera against phosphorylated Smad2 (pSmad2-Ser465/467, clone A5S; Millipore), Smad2/3 (Millipore), GAPDH (sc-32233; Santa Cruz Biotechnology), or β-actin (Sigma-Aldrich), using the SuperSignal West Pico Chemiluminescent Substrate (Pierce).
ELISA
Supernatants from M1 and M2 macrophages were tested for the presence of cytokines and growth factors using commercially available ELISA for TNF-α, IL-10, and IL-6 (all from ImmunoTools); IL-12 p40 (OptEIATM IL-12 p40 set; BD Pharmingen); and activin A (R&D Systems) following the protocols supplied by the manufacturers. Murine activin A was determined with the Quantikine Human/Mouse/Rat Activin A Immunoassay (R&D Systems).
Reporter gene assays
The FOLR2 gene promoter–based reporter construct pFOLR2-200Luc was generated by PCR amplification of the −210/+2 fragment of the FOLR2 promoter29 using oligonucleotides 5′-CCCAAGCTTCCCCCTGGTGATGAGCAATTC-3′ and 5′-CCGCTC GAGGCTCTGGTAAGCACTGAGTG-3′, and cloning the resulting fragment into HindIII/XhoI-digested pXP2. Mv1Lu cells, a well-established cellular model used to study signaling in the TGF-β superfamily,26 or murine embryo fibroblasts were transfected with 0.5 μg of the Smad2-dependent p3TP-Lux reporter construct30 or pFOLR2-200Luc, respectively, using SuperFect (QIAGEN). After transfections, cells were washed, cultured in DMEM plus 0.2% FCS, and treated with undiluted conditioned medium from M1 (GM-CSF) or M2 macrophages, 25 ng/mL of recombinant human activin A (Miltenyi Biotec), or 10 ng/mL of TGF-β1 (R&D Systems) for 24 hours. When required, cells were preincubated for 30 minutes with 10μM SB431542 (Sigma-Aldrich) before treatment. To neutralize activin A activity, 0.1 μg/mL blocking monoclonal antibody (R&D Systems) was used. In some experiments, cells were cotransfected with 0.4 μg of expression vector encoding a dominant-negative mutant of either Smad231 or Smad3.32 To normalize transfection efficiency, cells were cotransfected with an SV40 promoter–based β-galactosidase (β-gal) expression plasmid. Measurement of relative luciferase units and β-gal activity were performed using the Dual-Glo Luciferase Assay System (Promega) and the Galacto-Light kit (Tropix), respectively, in a Varioskan Flash spectral scanning multimode reader (Thermo Scientific).
qRT-PCR
Oligonucleotides for selected genes were designed using Roche software for quantitative real-time RT-PCR (qRT-PCR). Total RNA was extracted using the RNeasy kit (QIAGEN) and retrotranscribed, and individually amplified cDNA was quantified using the Universal Human Probe Library from Roche Diagnostics. Assays were done in triplicate and the results were normalized according to the expression levels of 18S rRNA and GAPDH RNA. Results were expressed using the ΔΔCT method for quantitation. All microarray data have been deposited in the Gene Expression Omnibus under accession number GSE27792.
Results
M1 macrophage–conditioned medium prevents the acquisition of FRβ expression
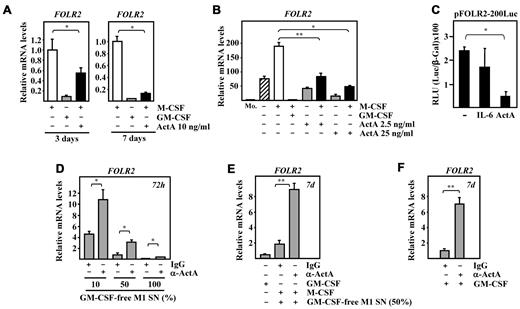
As reported previously, the expression of cell-surface FRβ, which is encoded by the FOLR2 gene, identifies IL-10–producing, ex vivo–isolated TAMs and in vitro–polarized M2 macrophages,22 but not M1 macrophages (Figure 1A). In fact, FRβ mediates folate-FITC capture by M2-polarized macrophages, but not by M1 macrophages (Figure 1B). GM-CSF and M-CSF oppositely modulated FOLR2 gene expression: GM-CSF greatly down-regulated FOLR2 RNA levels in FRβ-positive M2 macrophages (Figure 1C), whereas M1 macrophage–conditioned medium significantly inhibited FOLR2 RNA induction (Figure 1D). This latter inhibitory activity was observed when the M1 macrophage–conditioned medium was used either undiluted (P = .0009), diluted 1/2 (P = .001), or diluted 1/10 (more than 90% reduction; P = .001) (Figure 1D). Therefore, M1 macrophage–conditioned medium contains a factor(s) that prevents the acquisition of the M2-specific marker FRβ.
M1 macrophages inhibit FRβ expression on M2-polarized macrophages and release high levels of activin A. (A) Cell-surface expression of FRβ on M1 and M2 macrophages determined by flow cytometry using a rabbit polyclonal antiserum against human FRβ (empty histogram) or a nonspecific rabbit preimmune antiserum (filled histogram). The percentage of marker-positive cells and the mean fluorescence intensity (in parentheses) are indicated. (B) FRβ function in M1 and M2 macrophages demonstrated by confocal microscopy on cells incubated at 37°C with folate-FITC (green fluorescence) and Transferrin-Texas red (red fluorescence). (C) FOLR2 mRNA expression levels determined by qRT-PCR in M2 macrophages after replacement of the culture supernatant by either M-CSF (gray histograms)- or GM-CSF (empty histograms)–containing complete medium for 48 hours. Results are expressed as relative mRNA levels (relative to GAPDH RNA levels and referred to the RNA levels in cells maintained in M-CSF–containing medium). The means and SD of triplicate determinations are shown; *P < .005. (D) FOLR2 mRNA expression levels determined by qRT-PCR on peripheral blood monocytes (Mo.) and monocytes cultured for 72 hours in the absence (0) or presence (M1 SN [%]) of increasing percentages of M1 macrophage–conditioned medium. Results are expressed as relative mRNA levels (relative to GAPDH RNA levels and referred to levels detected in Mo.). The means and SD of triplicate determinations are shown; *P < .005. (E) Relative INHBA gene expression in M1 and M2 macrophages determined by microarray DNA analysis (empty histograms) and qRT-PCR (gray histograms). (F) INHBA mRNA expression levels determined by qRT-PCR on the indicated macrophages after replacement of their respective culture supernatant with fresh complete medium containing either M-CSF (gray histograms) or GM-CSF (empty histograms) for 48 hours. The means and SD of triplicate determinations are shown. Results are expressed as relative mRNA levels (relative to GAPDH RNA levels) and referred to the INHBA RNA levels in cells treated with M-CSF; *P < .005. (G) Activin A levels released by M1 and M2 macrophages from 6 independent donors determined by ELISA. (H) Determination of activin A release during the differentiation of M1 and M2 macrophages from 2 independent donors determined by ELISA on culture supernatants removed at the indicated time points. (I) Activin A levels released by M1 and M2 macrophages untreated (−) or stimulated with 10 or 100 ng/mL LPS for 24 hours, and either maintained in their conditioned medium (no wash) or in fresh culture medium (wash) during the LPS stimulation period. (J) Activin A levels released by murine M1 and M2 BMDM from 4 independent samples determined by ELISA. For panels G-J, the means and SD of triplicate determinations are shown; P < .005; **P < .05.
M1 macrophages inhibit FRβ expression on M2-polarized macrophages and release high levels of activin A. (A) Cell-surface expression of FRβ on M1 and M2 macrophages determined by flow cytometry using a rabbit polyclonal antiserum against human FRβ (empty histogram) or a nonspecific rabbit preimmune antiserum (filled histogram). The percentage of marker-positive cells and the mean fluorescence intensity (in parentheses) are indicated. (B) FRβ function in M1 and M2 macrophages demonstrated by confocal microscopy on cells incubated at 37°C with folate-FITC (green fluorescence) and Transferrin-Texas red (red fluorescence). (C) FOLR2 mRNA expression levels determined by qRT-PCR in M2 macrophages after replacement of the culture supernatant by either M-CSF (gray histograms)- or GM-CSF (empty histograms)–containing complete medium for 48 hours. Results are expressed as relative mRNA levels (relative to GAPDH RNA levels and referred to the RNA levels in cells maintained in M-CSF–containing medium). The means and SD of triplicate determinations are shown; *P < .005. (D) FOLR2 mRNA expression levels determined by qRT-PCR on peripheral blood monocytes (Mo.) and monocytes cultured for 72 hours in the absence (0) or presence (M1 SN [%]) of increasing percentages of M1 macrophage–conditioned medium. Results are expressed as relative mRNA levels (relative to GAPDH RNA levels and referred to levels detected in Mo.). The means and SD of triplicate determinations are shown; *P < .005. (E) Relative INHBA gene expression in M1 and M2 macrophages determined by microarray DNA analysis (empty histograms) and qRT-PCR (gray histograms). (F) INHBA mRNA expression levels determined by qRT-PCR on the indicated macrophages after replacement of their respective culture supernatant with fresh complete medium containing either M-CSF (gray histograms) or GM-CSF (empty histograms) for 48 hours. The means and SD of triplicate determinations are shown. Results are expressed as relative mRNA levels (relative to GAPDH RNA levels) and referred to the INHBA RNA levels in cells treated with M-CSF; *P < .005. (G) Activin A levels released by M1 and M2 macrophages from 6 independent donors determined by ELISA. (H) Determination of activin A release during the differentiation of M1 and M2 macrophages from 2 independent donors determined by ELISA on culture supernatants removed at the indicated time points. (I) Activin A levels released by M1 and M2 macrophages untreated (−) or stimulated with 10 or 100 ng/mL LPS for 24 hours, and either maintained in their conditioned medium (no wash) or in fresh culture medium (wash) during the LPS stimulation period. (J) Activin A levels released by murine M1 and M2 BMDM from 4 independent samples determined by ELISA. For panels G-J, the means and SD of triplicate determinations are shown; P < .005; **P < .05.
M1 macrophages secrete activin A, which down-regulates FOLR2 gene expression
To identify M1-derived factors that prevent FRβ induction, we searched for soluble factors preferentially produced by M1 macrophages. Gene-expression profiling22,23 revealed that expression of the INHBA gene, which codes for the inhibin βA subunit,10,11 is much higher in M1 than in M2 macrophages (log2 M1/M2 = 6.1; P = 5.3 × 10−8; Figure 1E), a difference further confirmed by qRT-PCR on independent samples (Figure 1E) and on polarization of either CD14++CD16− or CD14+CD16+ monocytes (data not shown). Unlike FOLR2, INHBA RNA expression was induced in M2 macrophages exposed to GM-CSF and abrogated in M1 macrophages upon replacement of their conditioned medium with M-CSF (P < .005; Figure 1F). In agreement with RNA data and because of its inducibility by GM-CSF,19 INHBA-encoded activin A levels were significantly higher in M1 macrophage–conditioned medium from 6 independent donors (P < .05; Figure 1G), and were continuously increased during the GM-CSF–dependent M1 differentiation/polarization process (Figure 1H). Although LPS increases circulating activin A levels in vivo,18 the differential production of activin A by M1 and M2 macrophages was maintained after LPS stimulation regardless of whether cells were maintained in their conditioned medium during LPS activation (Figure 1I). Similar results were observed in murine BMDMs: murine M1 BMDMs secreted significantly higher levels of activin A than M2 BMDMs (Figure 1J). These results indicate that activin A is differentially regulated by GM-CSF and M-CSF and that activin A expression is inversely correlated with FOLR2 gene expression.
To determine whether activin A affects FOLR2 gene expression, M2 polarization was accomplished in the presence of recombinant human activin A. The M-CSF–dependent acquisition of FOLR2 RNA expression was significantly and dose dependently reduced by activin A, an effect that could be observed both during (3 days) and at the end (7 days) of the polarization process (Figure 2A-B). In fact, activin A inhibited both the basal and the M-CSF–dependent up-regulation of FOLR2 RNA (Figure 2B). Further, whereas IL-6 had no effect, the activity of the FOLR2 gene proximal regulatory region was significantly reduced (P = .0008) by activin A (Figure 2C), indicating a transcriptional effect. Therefore, activin A inhibits the appearance of FOLR2 RNA in macrophages, and it can be concluded that the differential expression of FRβ on M1 and M2 macrophages derives at least in part from the opposite actions of GM-CSF and M-CSF on INHBA gene expression.
Activin A inhibits FRβ expression and mediates the inhibitory effect of GM-CSF or M1 macrophage–conditioned medium. (A) FOLR2 mRNA expression levels in macrophages differentiated for 3 days (left panel) or 7 days (right panel) in the presence of M-CSF, GM-CSF, or M-CSF + activin A (ActA; 10 ng/mL) determined by qRT-PCR. (B) FOLR2 mRNA expression levels determined by qRT-PCR on monocytes (Mo.) or macrophages cultured for 7 days in the presence of the indicated cytokine combinations. For panels A and B, results are expressed as mRNA levels relative to GAPDH RNA and referred to the expression level observed in the presence of M-CSF (A) or in monocytes (B). The means and SD of triplicate determinations are shown; *P < .005; **P < .05. (C) Transcriptional activity of the pFOLR2-200Luc reporter construct in Mv1Lu cells incubated in the absence (−) or presence of IL-6 or activin A (ActA; 25 μg/mL). For normalization purposes, cells were cotransfected with the Rous Sarcoma Virus promoter–β-gal expression plasmid, and results are presented as RLU (relative light units), which indicate the units of luciferase activity per unit of β-gal activity for each assay condition. The means and SD of triplicate determinations are shown; *P < .005. (D) FOLR2 mRNA expression levels determined by qRT-PCR on monocytes exposed for 72 hours to increasing concentrations of GM-CSF–free M1 macrophage–conditioned medium (GM-CSF–free M1 SN %) and in the presence of 100 ng/mL of a blocking anti–activin A antibody (α-ActA) or an isotype-matched antibody (IgG). Results are expressed as relative mRNA levels (relative to GAPDH RNA levels). The means and SD of triplicate determinations are shown; *P < .005. (E) FOLR2 mRNA expression levels determined by qRT-PCR on M1 macrophages or M2 macrophages generated in the presence of GM-CSF–free M1 macrophage–conditioned medium (50%) and with the daily addition of a blocking anti–activin A antibody (α-ActA) or an isotype-matched antibody (IgG). Results are expressed as relative mRNA levels (relative to GAPDH RNA levels). The means and SD of triplicate determinations are shown; **P < .05. (F) FOLR2 mRNA expression levels determined by qRT-PCR on M1 macrophages generated in the presence of a blocking anti–activin A antibody (α-ActA) or an isotype-matched antibody (IgG). Results are expressed as relative mRNA levels (relative to GAPDH RNA levels). The means and SD of triplicate determinations are shown; **P < .05.
Activin A inhibits FRβ expression and mediates the inhibitory effect of GM-CSF or M1 macrophage–conditioned medium. (A) FOLR2 mRNA expression levels in macrophages differentiated for 3 days (left panel) or 7 days (right panel) in the presence of M-CSF, GM-CSF, or M-CSF + activin A (ActA; 10 ng/mL) determined by qRT-PCR. (B) FOLR2 mRNA expression levels determined by qRT-PCR on monocytes (Mo.) or macrophages cultured for 7 days in the presence of the indicated cytokine combinations. For panels A and B, results are expressed as mRNA levels relative to GAPDH RNA and referred to the expression level observed in the presence of M-CSF (A) or in monocytes (B). The means and SD of triplicate determinations are shown; *P < .005; **P < .05. (C) Transcriptional activity of the pFOLR2-200Luc reporter construct in Mv1Lu cells incubated in the absence (−) or presence of IL-6 or activin A (ActA; 25 μg/mL). For normalization purposes, cells were cotransfected with the Rous Sarcoma Virus promoter–β-gal expression plasmid, and results are presented as RLU (relative light units), which indicate the units of luciferase activity per unit of β-gal activity for each assay condition. The means and SD of triplicate determinations are shown; *P < .005. (D) FOLR2 mRNA expression levels determined by qRT-PCR on monocytes exposed for 72 hours to increasing concentrations of GM-CSF–free M1 macrophage–conditioned medium (GM-CSF–free M1 SN %) and in the presence of 100 ng/mL of a blocking anti–activin A antibody (α-ActA) or an isotype-matched antibody (IgG). Results are expressed as relative mRNA levels (relative to GAPDH RNA levels). The means and SD of triplicate determinations are shown; *P < .005. (E) FOLR2 mRNA expression levels determined by qRT-PCR on M1 macrophages or M2 macrophages generated in the presence of GM-CSF–free M1 macrophage–conditioned medium (50%) and with the daily addition of a blocking anti–activin A antibody (α-ActA) or an isotype-matched antibody (IgG). Results are expressed as relative mRNA levels (relative to GAPDH RNA levels). The means and SD of triplicate determinations are shown; **P < .05. (F) FOLR2 mRNA expression levels determined by qRT-PCR on M1 macrophages generated in the presence of a blocking anti–activin A antibody (α-ActA) or an isotype-matched antibody (IgG). Results are expressed as relative mRNA levels (relative to GAPDH RNA levels). The means and SD of triplicate determinations are shown; **P < .05.
To evaluate the involvement of activin A in the inhibitory effects that GM-CSF and M1 macrophage–conditioned medium have on FOLR2 mRNA expression, a blocking anti–activin A antibody was used in 3 different experimental settings. First, the inhibitory action of different doses of GM-CSF–free M1 macrophage–conditioned medium on FOLR2 mRNA up-regulation in cultured monocytes was significantly reversed in the presence of the blocking anti–activin A antibody (Figure 2D). Second, antibody-mediated blockade of activin A also significantly reduced the inhibitory activity of GM-CSF–free M1 macrophage–conditioned medium on the acquisition of FOLR2 mRNA by M2 macrophages (Figure 2E). Finally, and most importantly, the presence of the anti–activin A antibody during the GM-CSF–dependent M1 macrophage polarization also led to significantly higher levels of FOLR2 mRNA (Figure 2F). These results indicate that the inhibitory effect of GM-CSF and the GM-CSF–free M1 macrophage–conditioned medium on the expression of the prototypical M2 marker FOLR2 is at least in part mediated by activin A, suggesting a role for this member of the TGFβ family in macrophage polarization.
Activin A contributes to M1 macrophage polarization
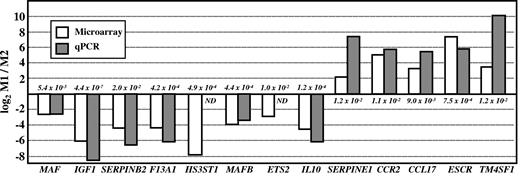
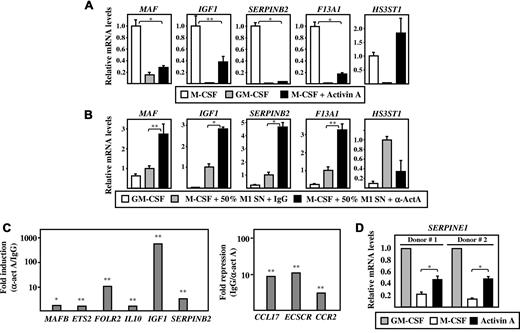
Considering the above results, we assessed the influence of activin A on genes that, like FOLR2, are differentially expressed between M1 and M2 macrophages (Figure 3).22,23,33 Regarding M2-specific markers, after the 7-day polarization process, the presence of activin A led to a reduction in the M-CSF–dependent induction of MAF, IGF1 and F13A1, blunted that of SERPINB2, but had no inhibitory effect on HS3ST1 expression (Figure 4A). Moreover, the presence of a blocking anti–activin A antibody increased the expression of MAF, IGF1, F13A1, and SERPINB2 mRNA yielded on polarization in the presence of GM-CSF–free M1 macrophage–conditioned medium (Figure 4B). As a final proof of the involvement of activin A in macrophage polarization, the GM-CSF–dependent polarization of M1 macrophages was carried out in the presence of the blocking anti–activin A antibody. As shown in Figure 4C, the expression of the M2 (M-CSF)–specific markers MAFB, ETS2, FOLR2, IL10, IGF1, and SERPINB2 was significantly enhanced on blockade of activin A activity during the polarization process. Furthermore, the presence of the antibody also led to a significant reduction of 3 M1 (GM-CSF)–specific markers: CCL17, ECSCR, and CCR2 (Figure 4C). These results indicate that activin A plays a relevant role in the GM-CSF–mediated M1 macrophage polarization, because it potentiates the expression of M1 (GM-CSF)–specific markers and inhibits that of M2 (M-CSF) markers. Therefore, considering its constitutive expression during GM-CSF–mediated M1 polarization, it is tempting to speculate that activin A shapes the polarization of macrophages in an autocrine/paracrine manner.
Relative expression of the indicated genes in M1 and M2 macrophages. Gene expression was determined by microarray DNA analysis (empty histograms) and qRT-PCR (gray histograms). The adjusted P value for the microarray data of each specific gene is indicated.
Relative expression of the indicated genes in M1 and M2 macrophages. Gene expression was determined by microarray DNA analysis (empty histograms) and qRT-PCR (gray histograms). The adjusted P value for the microarray data of each specific gene is indicated.
Effect of activin A on the acquisition of M1- and M2-specific markers. (A) MAF, IGF1, SERPINB2, F13A1, and HS3ST1 mRNA expression levels determined by qRT-PCR on macrophages differentiated for 7 days in M-CSF, GM-CSF, or M-CSF plus activin A (10 ng/mL). (B) MAF, IGF1, SERPINB2, F13A1, and HS3ST1 mRNA expression levels determined by qRT-PCR on macrophages differentiated for 7 days in GM-CSF or M-CSF plus GM-CSF–free M1-conditioned medium together with either a blocking anti–activin A antibody (M-CSF + 50% M1 SN + α-ActA) or an isotype-matched antibody (M-CSF + 50% M1 SN + IgG). (C) Expression of the indicated genes in M1 macrophages generated in the presence of a blocking anti–activin A antibody (100 ng/mL) and relative to their expression in M1 macrophages generated in the presence of an isotype-matched antibody (IgG) determined by qRT-PCR using microfluidic cards. The experiment was performed in triplicate and data are presented on a log scale; *P < .005; **P < .05. The left panel shows the expression of M2 (M-CSF)–specific markers, and these results as expressed relative to the level of expression of each gene in the presence of the isotype-matched antibody (Fold induction α-ActA/IgG). The right panel shows the expression of M1 (GM-CSF)–specific markers and these results are expressed relative to the level of expression of each gene in the presence of the anti–activin A antibody (Fold repression, IgG/α-ActA). (D) SERPINE1 mRNA expression levels determined by qRT-PCR on macrophages from 2 independent donors and treated for 7 days with M-CSF, GM-CSF, or activin A. Results are expressed as relative mRNA levels (relative to GAPDH RNA levels) and refer to the expression level observed in the presence of M-CSF (A), M-CSF + 50% M1 SN + IgG (B), or GM-CSF (D). The means and SD of triplicate determinations are shown; *P < .005; **P < .05.
Effect of activin A on the acquisition of M1- and M2-specific markers. (A) MAF, IGF1, SERPINB2, F13A1, and HS3ST1 mRNA expression levels determined by qRT-PCR on macrophages differentiated for 7 days in M-CSF, GM-CSF, or M-CSF plus activin A (10 ng/mL). (B) MAF, IGF1, SERPINB2, F13A1, and HS3ST1 mRNA expression levels determined by qRT-PCR on macrophages differentiated for 7 days in GM-CSF or M-CSF plus GM-CSF–free M1-conditioned medium together with either a blocking anti–activin A antibody (M-CSF + 50% M1 SN + α-ActA) or an isotype-matched antibody (M-CSF + 50% M1 SN + IgG). (C) Expression of the indicated genes in M1 macrophages generated in the presence of a blocking anti–activin A antibody (100 ng/mL) and relative to their expression in M1 macrophages generated in the presence of an isotype-matched antibody (IgG) determined by qRT-PCR using microfluidic cards. The experiment was performed in triplicate and data are presented on a log scale; *P < .005; **P < .05. The left panel shows the expression of M2 (M-CSF)–specific markers, and these results as expressed relative to the level of expression of each gene in the presence of the isotype-matched antibody (Fold induction α-ActA/IgG). The right panel shows the expression of M1 (GM-CSF)–specific markers and these results are expressed relative to the level of expression of each gene in the presence of the anti–activin A antibody (Fold repression, IgG/α-ActA). (D) SERPINE1 mRNA expression levels determined by qRT-PCR on macrophages from 2 independent donors and treated for 7 days with M-CSF, GM-CSF, or activin A. Results are expressed as relative mRNA levels (relative to GAPDH RNA levels) and refer to the expression level observed in the presence of M-CSF (A), M-CSF + 50% M1 SN + IgG (B), or GM-CSF (D). The means and SD of triplicate determinations are shown; *P < .005; **P < .05.
Given the inhibitory action of an anti–activin A antibody on the acquisition of M1 markers, and because activin A is proposed to be a Th2-polarizing cytokine,21 its effects on the expression of genes preferentially found in both M1- and IL-4–activated macrophages33 were also evaluated. Monocytes were treated with activin A for 72 hours in the absence of GM-CSF. Activin A treatment did not modify the expression of TM4SF1, CCL17, or ILR1N, the latter being a known activin A target gene14 (supplemental Figure 2). However, activin A significantly enhanced SERPINE1 RNA levels (Figure 4D), the expression of which was significantly higher in M1 than in M2 macrophages (Figure 3). Therefore, whereas activin A contributed to GM-CSF–mediated polarization, it was also capable by itself of up-regulating the expressing of genes preferentially found in M1 macrophages, further confirming its ability to shape the phenotypic polarization of macrophages.
Activin A mediates the growth-inhibitory activity of M1 macrophage–conditioned medium
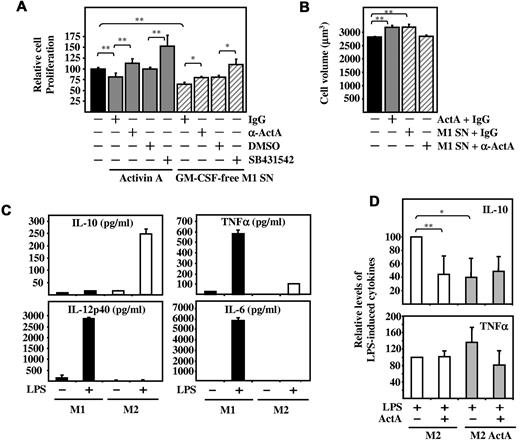
Although originally identified as gonadally derived regulators of pituitary follicle-stimulating hormone, activin A exerts multiple actions, including cancer cell growth arrest.10,13,34,35 The tumor-suppressive properties of activin A prompted us to analyze whether M1 macrophage–conditioned medium affected K562 leukemic cell growth, a function previously ascribed to activin A.36 GM-CSF–free M1 macrophage–conditioned medium significantly inhibited the growth of K562 leukemic cells to a greater extent than recombinant activin A (25 ng/mL), and this growth-suppressive activity was significantly reverted in the presence of either a blocking anti–activin A monoclonal antibody or SB431542, an ALK4/5/7 inhibitor known to prevent activin A–induced Smad2/3 phosphorylation37 (Figure 5A). Moreover, in agreement with previous results,36 GM-CSF–free M1-conditioned medium increased K562 cell volume, a feature associated with differentiation into hemoglobin-expressing cells, and this differentiation-inducing ability was reduced by a blocking anti–activin A monoclonal antibody (Figure 5B). These results indicate that M1 macrophages release functional activin A, endowing them with tumor-resistance capability, a defining property of M1-polarized macrophages.38
Effects of activin A on M1 macrophage effector functions. Panels A and B show growth-inhibitory activity. (A) Proliferation of K562 cells exposed for 96 hours to activin A (25 ng/mL) or to GM-CSF–free M1 macrophage–conditioned medium (GM-CSF–free M1 SN) in the presence of a blocking anti–activin A antibody (α-ActA, 100 ng/mL), an isotype-matched antibody (IgG, 100 ng/mL), 10 μM SB431542, or a similar amount of vehicle (DMSO) as a control. Results show the means and SD of the effects of 3 independent preparations of GM-CSF–free M1 (GM-CSF)–conditioned medium and are expressed relative to the proliferation measured in untreated cells (Relative cell proliferation). (B) Cell volume of K562 cells after exposure for 96 hours to either activin A (25 ng/mL) or to GM-CSF–free M1 macrophage–conditioned medium (M1 SN) in the absence or presence of a blocking anti–activin A monoclonal antibody. The means and SD of triplicate determinations are shown; *P < .005; **P < .05. Panels C and D show the LPS-induced cytokine profile. (C) Determination of IL-12p40, IL-6, IL-10, and TNFα release by ELISA in culture supernatants of M1 and M2 macrophages either untreated or stimulated with LPS (10 ng/mL) for 24 hours. The means and SD of triplicate determinations are shown. (D) Determination of IL-10 and TNFα release by ELISA in the culture supernatant of M2 macrophages differentiated in the absence (M2) or presence of activin A (M2 ActA) either unstimulated or stimulated with LPS (10 ng/mL) for 24 hours in the presence or absence of activin A. The means and SD of triplicate determinations are shown; *P < .005; **P < .05.
Effects of activin A on M1 macrophage effector functions. Panels A and B show growth-inhibitory activity. (A) Proliferation of K562 cells exposed for 96 hours to activin A (25 ng/mL) or to GM-CSF–free M1 macrophage–conditioned medium (GM-CSF–free M1 SN) in the presence of a blocking anti–activin A antibody (α-ActA, 100 ng/mL), an isotype-matched antibody (IgG, 100 ng/mL), 10 μM SB431542, or a similar amount of vehicle (DMSO) as a control. Results show the means and SD of the effects of 3 independent preparations of GM-CSF–free M1 (GM-CSF)–conditioned medium and are expressed relative to the proliferation measured in untreated cells (Relative cell proliferation). (B) Cell volume of K562 cells after exposure for 96 hours to either activin A (25 ng/mL) or to GM-CSF–free M1 macrophage–conditioned medium (M1 SN) in the absence or presence of a blocking anti–activin A monoclonal antibody. The means and SD of triplicate determinations are shown; *P < .005; **P < .05. Panels C and D show the LPS-induced cytokine profile. (C) Determination of IL-12p40, IL-6, IL-10, and TNFα release by ELISA in culture supernatants of M1 and M2 macrophages either untreated or stimulated with LPS (10 ng/mL) for 24 hours. The means and SD of triplicate determinations are shown. (D) Determination of IL-10 and TNFα release by ELISA in the culture supernatant of M2 macrophages differentiated in the absence (M2) or presence of activin A (M2 ActA) either unstimulated or stimulated with LPS (10 ng/mL) for 24 hours in the presence or absence of activin A. The means and SD of triplicate determinations are shown; *P < .005; **P < .05.
Activin A modulates the cytokine profile of M2 macrophages
In addition to their opposite responses toward tumor cells, M1 and M2 macrophages differ in T-cell–stimulatory ability and pathogen-stimulated cytokine/chemokine profile.6,7 In allogeneic MLRs, M1 macrophages induced considerable higher T-cell proliferation than M2 macrophages, but exposure of the latter to activin A did not modify their T cell–stimulatory ability (supplemental Figure 3), a result reminiscent of the lack of effect of follistatin on the allostimulatory function of monocyte-derived dendritic cells.20 Regarding cytokine release, and in agreement with previous reports,7 LPS stimulation of M1 macrophages led to the production of the proinflammatory cytokines TNFα, IL-12p40, and IL-6 (Figure 5C) and to the acquisition of dendritic cell maturation ability (supplemental Figure 4), whereas LPS-stimulated M2 macrophages produced high levels of IL-10 (Figure 5C). Activin A also significantly reduced the release of IL-10 from LPS-stimulated M2 macrophages (P = .01), but had no effect on the production of TNFα (Figure 5D). Moreover, macrophages exposed to activin A during M-CSF–driven polarization showed a diminished production of IL-10 in response to LPS (P = .003; Figure 5D). Therefore, and in agreement with its effect on IL10 mRNA levels during macrophage polarization (Figure 4C), activin A negatively regulates IL-10 production from M2 macrophages and skews macrophage polarization by impairing the acquisition of the anti-inflammatory phenotype and cytokine profile.
Activin A influences macrophage polarization via Smad2/3 activation
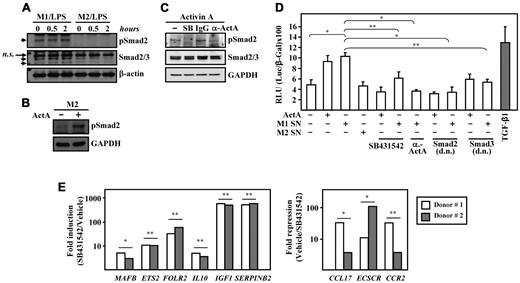
Because activin A triggers Smad2 phosphorylation,13 we next determined the extent of Smad2 activation in unstimulated and LPS-stimulated macrophages. Smad2 was found to be constitutively phosphorylated in M1 macrophages and remained activated after LPS stimulation (Figure 6A). In contrast, no Smad2 phosphorylation was detected in either untreated or LPS-stimulated M2 macrophages (Figure 6A). The absence of Smad2 activation in M2 macrophages was not due to a defective activin/Smad signaling pathway, because activin A treatment of M2 macrophages led to overt Smad2 phosphorylation (Figure 6B). As proof of the specificity of the anti–activin A antibody used in the preceding experiments, the presence of the antibody reduced the degree of Smad2 phosphorylation triggered by activin A on M2 macrophages (Figure 6C), whereas the activin A-induced Smad2 phosphorylation was completely prevented in the presence of SB431542, which prevents Smad2/3 phosphorylation37 (Figure 6C).
Smad2 is constitutively phosphorylated in M1 macrophages, in which activin A release activates Smad-dependent reporter genes. (A) Detection of phosphorylated Smad2 and total Smad2/3 in lysates of untreated or LPS-treated M1 and M2 macrophages determined by Western blot analysis. β-actin expression levels were determined in parallel as a loading control. The bands corresponding to Smad2 and Smad3 are indicated by arrowheads (n.s., indicates nonspecific band). (B) Detection of activated Smad2 in lysates of untreated or activin A–treated M2 macrophages determined by Western blot analysis. GAPDH expression levels were determined in parallel as a loading control. (C) Detection of activated Smad2 by Western blot analysis on lysates of M2 macrophages subjected to a 30-minute treatment with DMSO (−), SB431542 (SB), an anti–activin A antibody (α-ActA), or an isotype-matched antibody (IgG), and then treated with activin A for 1 hour. Total Smad2/3 and GAPDH expression levels were determined in parallel as loading controls. (D) Transcriptional activity of the p3TP-Lux reporter construct in Mv1Lu cells either unstimulated or exposed to 10 ng/mL of TGFβ1, 25 ng/mL of activin A, or conditioned medium from M1 (M1 SN) or M2 (M2 SN) macrophages. Where indicated, cells were preincubated for 30 minutes with 10μM SB431542 before treatment, maintained in culture medium with 0.1 μg/mL blocking antibody against activin A (anti-ActA), or cotransfected with expression vectors coding for dominant-negative mutants of either Smad2 (Smad2, d.n.) or Smad3 (Smad3, d.n.). For normalization purposes, cells were cotransfected with the Rous Sarcoma Virus promoter–β-gal expression plasmid, and results are presented as RLU (relative light units), which indicate the units of luciferase activity per unit of β-gal activity for each assay condition. The means and SD of triplicate determinations are shown. Six replicas of each experiment were performed and means and SD are shown; *P < .005; **P < .05. (E) Expression of the indicated genes in M1 macrophages generated in the presence of SB431542 (10μM), and compared with their expression in M1 macrophages generated without the inhibitor (Vehicle, DMSO) determined by qRT-PCR using microfluidic cards. The experiment was performed in triplicate on macrophages from 2 independent donors and data are presented on a log scale; *P < .005; **P < .05. The left panel shows the expression of M2 (M-CSF)–specific markers, and these results as expressed relative to the level of expression of each gene in the presence of DMSO (Fold induction SB431542/Vehicle). The right panel shows the expression of M1 (GM-CSF)–specific markers, and these results as expressed relative to the level of expression of each gene in the presence of the SB431542 inhibitor (Fold repression, Vehicle/SB431542).
Smad2 is constitutively phosphorylated in M1 macrophages, in which activin A release activates Smad-dependent reporter genes. (A) Detection of phosphorylated Smad2 and total Smad2/3 in lysates of untreated or LPS-treated M1 and M2 macrophages determined by Western blot analysis. β-actin expression levels were determined in parallel as a loading control. The bands corresponding to Smad2 and Smad3 are indicated by arrowheads (n.s., indicates nonspecific band). (B) Detection of activated Smad2 in lysates of untreated or activin A–treated M2 macrophages determined by Western blot analysis. GAPDH expression levels were determined in parallel as a loading control. (C) Detection of activated Smad2 by Western blot analysis on lysates of M2 macrophages subjected to a 30-minute treatment with DMSO (−), SB431542 (SB), an anti–activin A antibody (α-ActA), or an isotype-matched antibody (IgG), and then treated with activin A for 1 hour. Total Smad2/3 and GAPDH expression levels were determined in parallel as loading controls. (D) Transcriptional activity of the p3TP-Lux reporter construct in Mv1Lu cells either unstimulated or exposed to 10 ng/mL of TGFβ1, 25 ng/mL of activin A, or conditioned medium from M1 (M1 SN) or M2 (M2 SN) macrophages. Where indicated, cells were preincubated for 30 minutes with 10μM SB431542 before treatment, maintained in culture medium with 0.1 μg/mL blocking antibody against activin A (anti-ActA), or cotransfected with expression vectors coding for dominant-negative mutants of either Smad2 (Smad2, d.n.) or Smad3 (Smad3, d.n.). For normalization purposes, cells were cotransfected with the Rous Sarcoma Virus promoter–β-gal expression plasmid, and results are presented as RLU (relative light units), which indicate the units of luciferase activity per unit of β-gal activity for each assay condition. The means and SD of triplicate determinations are shown. Six replicas of each experiment were performed and means and SD are shown; *P < .005; **P < .05. (E) Expression of the indicated genes in M1 macrophages generated in the presence of SB431542 (10μM), and compared with their expression in M1 macrophages generated without the inhibitor (Vehicle, DMSO) determined by qRT-PCR using microfluidic cards. The experiment was performed in triplicate on macrophages from 2 independent donors and data are presented on a log scale; *P < .005; **P < .05. The left panel shows the expression of M2 (M-CSF)–specific markers, and these results as expressed relative to the level of expression of each gene in the presence of DMSO (Fold induction SB431542/Vehicle). The right panel shows the expression of M1 (GM-CSF)–specific markers, and these results as expressed relative to the level of expression of each gene in the presence of the SB431542 inhibitor (Fold repression, Vehicle/SB431542).
Further evidence for the role of activin A in shaping M1 macrophage polarization was obtained through evaluation of the transcriptional effects of M1 macrophage–derived activin A. Like recombinant TGFβ1 and activin A, M1-conditioned medium transactivated the p3TP-Lux reporter construct (P = .0008), whereas supernatants from M2 macrophages had no effect (Figure 6D). The transactivation ability of the M1 macrophage–conditioned medium was abolished by: (1) SB431542 (P = .015; Figure 6D); (2) cotransfection of dominant-negative forms of Smad2 (P = .0018) or Smad3 (P = .0055; Figure 6D); and (3) the blocking anti–activin A antibody (P = .0001; Figure 6D). These results demonstrate that M1-derived activin A activates Smad2/3 in an autocrine/paracrine manner, thus providing molecular support for its capacity to shape macrophage polarization. Further, because the transactivation ability of the M1 macrophage–conditioned medium was completely abolished by an anti–activin A antibody, the basal phosphorylation state of Smad2/3 in M1 macrophages must reflect an autocrine/paracrine effect of activin A.
Smad signaling modulates macrophage polarization
The differential status of the basal Smad2/3 phosphorylation between M1 and M2 macrophages prompted us to study the impact of Smad2/3 signaling on macrophage polarization. M1 macrophages were generated in the presence of the ALK4/5/7 inhibitor SB431542. As expected, blockade of Smad2/3 activation resulted in a robust increase in FOLR2 expression (> 30-fold; Figure 6E), a finding compatible with the inhibitory effect of activin A on FOLR2 levels (Figure 2A-B and Figure 4C). The resulting macrophage population also exhibited higher expression of other M2-specific markers,23,33 including IGF1 (> 500-fold), SERPINB2 (> 500-fold), MAFB, MAF, and ETS2 (all 10-fold), with the latter known to determine the transcriptional profile of TAMs39 (Figure 6E). In contrast, the presence of SB431542 led to significantly lower levels of the M1-specific markers CCL17, ECSCR, and CCR2 (Figure 6E), a result that is in agreement with the sensitivity of these 3 genes to the presence of the blocking anti–activin A antibody during the GM-CSF–driven M1 polarization (Figure 4C). A higher level of IL10 mRNA, the presence of which is a defining property of M2-polarized macrophages,7 was also observed when polarization took place in the presence of SB431542 (Figure 6E). These results suggest that the activin A-triggered Smad signaling pathway has an important role in macrophage polarization, because both activin A blockade and inhibition of Smad2/3 activation shift the macrophage gene signature toward the appearance of an anti-inflammatory phenotype, thus posing activin A as a critical determinant for macrophage polarization.
Discussion
Macrophage differentiation and polarization are critically determined by the cellular environment, which also dictates cytokine responsiveness.40 The search for the mechanisms underlying GM-CSF– and M-CSF–driven macrophage differentiation and the acquisition of anti-inflammatory M2 macrophage markers has led to the identification of activin A as a factor that shapes macrophage polarization in response to GM-CSF, limits the production of IL-10, contributes to the expression of M1 macrophage polarization, and prevents the expression of M2-specific markers. The relevance of the activin A expression for macrophage polarization is underscored by the opposite effect that ascitic fluids have on INHBA gene expression and the levels of M2-specific markers (data not shown). In addition, considering the ability of macrophages to repolarize,41 activin A may halt the macrophage switch between distinct polarization states. To our knowledge, this is the first report describing the relevance of the activin A–Smad2/3 axis in human macrophage polarization and its involvement in preventing the acquisition of the phenotype and effector functions commonly associated with M2/anti-inflammatory macrophages.
The shift in macrophage polarization is now recognized as a relevant event in tumorigenesis, wound healing, and resolution of inflammation, and its deregulation underlies both tumor progression and chronic inflammatory diseases.41,42 Consequently, determination of the molecular mechanisms for macrophage polarization is a very active area of research and could result in the identification of novel opportunities for manipulating immune and inflammatory responses. The transcriptional basis for the various macrophage polarization states is beginning to be unraveled. Whereas NFκB and AP1 drive classic M1 macrophage polarization, the alternative M2 state is controlled by STAT6, peroxisome proliferator–activated receptorγ,42 STAT1, and NFκB p50.43 In the case of murine macrophages, type I interferon signaling appears to be essential for phenotypic and functional M2 polarization of BMDM in response to M-CSF25 and, more recently, the Jmjd3-Irf4 axis has been determined to be crucial for the expression of genes related to M2 macrophage polarization in BMDM exposed to M-CSF.44 Given the phenotypic differences between murine and human M2 macrophages in response to IL-4,45 it cannot be anticipated whether activin A and Smad signaling contribute to murine macrophage polarization. However, the much higher level of activin A produced by GM-CSF–polarized M1 BMDM (Figure 2F) would favor the hypothesis that activin A also modulates murine macrophage polarization.
Tumor-derived factors modulate macrophage polarization and contribute to the antitumor (M1)–to–protumor (M2) shift that takes place in TAMs during tumor progression.38 From this point of view, the preferential expression of activin A by M1 macrophages is compatible with its proapoptotic, tumor-suppressive, and anti-angiogenic effects,10,46,47 as well as with its capacity to inhibit proliferation of human tumor cells from various origins.10 Therefore, tumor cells that inhibit activin A activity or expression in macrophages (thus reducing their M1 phenotype signature) would exhibit a growth advantage, a property commonly ascribed to tumor cells promoting the acquisition of an M2-phenotypic signature.41 The fact that ascitic fluids from tumors of different origins oppositely modulate INHBA mRNA levels and M2 (M-CSF)–associated markers (data not shown) supports the validity of activin A as a marker for M1-polarized macrophages. On the other hand, the identification of various M2 (M-CSF)–specific markers with expression that is prevented or down-modulated by activin A (Figure 4) implies that activin A negatively affects the acquisition of the macrophage anti-inflammatory gene profile. This effect is particularly relevant in the case of IL-10, the release of which constitutes a hallmark of stimulated M2 macrophages.7 In fact, IL10 gene transcription in macrophages is dependent on the cMAF transcription factor,48 the expression of which is associated with M2 (M-CSF) polarization and in which RNA levels are reduced by activin A (Figure 4) and enhanced by interference with the Smad signaling pathway (data not shown). Consequently, the capacity of activin A to impair MAF RNA expression might underlie its negative regulatory effect on IL10 gene expression.
The ability of activin A to trigger arginase-1 expression and inhibit IFNγ-induced NO synthase 1 expression has led to the suggestion that it functions as a Th2 cytokine that promotes alternative murine macrophage activation.21 However, in the case of human macrophages, although M1 macrophages release high levels of activin A, they do not display any of the phenotypic markers that characterize alternatively activated human macrophages33 (Puig-Kröger, Sierra-Filardi, Vega, and Corbí, unpublished data). Activin A, which is synthesized by monocytes/macrophages in response to LPS,18 TNFα, or IL-1β,49 exhibits both pro- and anti-inflammatory activities18 because it stimulates the production of IL-1 and TNFα by human monocytes and macrophages and inhibits IL-10 effects on prostatic epithelial cells.50 In fact, activin A blockade by follistatin leads to reduced levels of LPS-induced IL-1 and TNFα.18 The link between proinflammatory macrophage polarization and activin A is further illustrated by the fact that activin A expression is inhibited by anti-inflammatory agents such as glucorticoids and retinoic acid.19,20 Because factors promoting M1/classic macrophage polarization enhance activin A production, its expression appears to be a common parameter of M1-polarized macrophages, as well as a critical contributor to their phenotype and effector functions. In this regard, the expression of type I (ALK4, ACVR1B) and type II (ACVR2A, ACVR2B) activin receptor mRNA in M1 macrophages (data not shown) explains the ability of activin A to influence the gene-expression profile of M1-polarized macrophages in a paracrine/autocrine manner. This appears to be true because: (1) Smad2 is constitutively phosphorylated in M1 macrophages and (2) polarization in the presence of a blocking anti–activin A antibody or the ALK4/5/7 inhibitor SB431542 not only leads to up-regulation/induction of M2-specific makers, but also impairs the expression of genes associated with GM-CSF–driven M1 polarization. Therefore, activin A (like other TGFβ family members) has a prominent role in determining the macrophage polarization state, and consequently in shaping the inflammatory response of macrophages to exogenous stimuli.
In summary, the results of the present study identify activin A as a relevant contributor to the differential gene-expression profiles and effector functions exhibited by proinflammatory and anti-inflammatory macrophages. The importance of activin A in macrophage polarization is supported by its ability to reduce IL-10 production by anti-inflammatory macrophages and to inhibit IL-10–producing ability during M-CSF–driven polarization. The identification of a set of M2 macrophage–specific genes found to be expressed in TAMs22 and to be negatively affected by activin A provides novel potential therapeutic targets for the modulation of the macrophage inflammatory response under pathologic conditions.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge Dr Carmen Sánchez-Torres for valuable discussions and suggestions, Drs Miguel Relloso and Sonia Chamorro for help with murine macrophage generation, and Dr José Alberto García-Sanz for valuable help with flow cytometry.
This work was supported by the Ministerio de Ciencia e Innovación (grant BFU2008-01493-BMC), Genoma España (MEICA project), Instituto de Salud Carlos III (Spanish Network for the Research in Infectious Diseases REIPI RD06/0008, and Red de Investigación en SIDA RIS RD06/0006/1016), Fundación para la Investigación y Prevención del SIDA en España (FIPSE 36663/07), and Fundación Mutua Madrileña to A.L.C.; by grant SAF2007-61827 to C.B., and by grant PI08/1208 from Instituto de Salud Carlos III to A.P.-K. A.P.-K. is also supported by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III (CP06/00199). The CIBER de Enfermedades Raras (CIBERER) is an initiative of the Instituto de Salud Carlos III of Spain.
Authorship
Contribution: E.S.-F., A.P.-K., F.J.B., and C.N. performed research and analyzed data; R.B. and M.I.P. contributed vital new reagents; M.A.V. analyzed data; C.B. and A.L.C. designed research and analyzed data; and A.L.C. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Angel L. Corbí, Centro de Investigaciones Biológicas, CSIC, Ramiro de Maeztu, 9 Madrid 28040 Spain; e-mail: acorbi@cib.csic.es.
References
Author notes
E.S.-F. and A.P.-K. contributed equally to this study.

![Figure 1. M1 macrophages inhibit FRβ expression on M2-polarized macrophages and release high levels of activin A. (A) Cell-surface expression of FRβ on M1 and M2 macrophages determined by flow cytometry using a rabbit polyclonal antiserum against human FRβ (empty histogram) or a nonspecific rabbit preimmune antiserum (filled histogram). The percentage of marker-positive cells and the mean fluorescence intensity (in parentheses) are indicated. (B) FRβ function in M1 and M2 macrophages demonstrated by confocal microscopy on cells incubated at 37°C with folate-FITC (green fluorescence) and Transferrin-Texas red (red fluorescence). (C) FOLR2 mRNA expression levels determined by qRT-PCR in M2 macrophages after replacement of the culture supernatant by either M-CSF (gray histograms)- or GM-CSF (empty histograms)–containing complete medium for 48 hours. Results are expressed as relative mRNA levels (relative to GAPDH RNA levels and referred to the RNA levels in cells maintained in M-CSF–containing medium). The means and SD of triplicate determinations are shown; *P < .005. (D) FOLR2 mRNA expression levels determined by qRT-PCR on peripheral blood monocytes (Mo.) and monocytes cultured for 72 hours in the absence (0) or presence (M1 SN [%]) of increasing percentages of M1 macrophage–conditioned medium. Results are expressed as relative mRNA levels (relative to GAPDH RNA levels and referred to levels detected in Mo.). The means and SD of triplicate determinations are shown; *P < .005. (E) Relative INHBA gene expression in M1 and M2 macrophages determined by microarray DNA analysis (empty histograms) and qRT-PCR (gray histograms). (F) INHBA mRNA expression levels determined by qRT-PCR on the indicated macrophages after replacement of their respective culture supernatant with fresh complete medium containing either M-CSF (gray histograms) or GM-CSF (empty histograms) for 48 hours. The means and SD of triplicate determinations are shown. Results are expressed as relative mRNA levels (relative to GAPDH RNA levels) and referred to the INHBA RNA levels in cells treated with M-CSF; *P < .005. (G) Activin A levels released by M1 and M2 macrophages from 6 independent donors determined by ELISA. (H) Determination of activin A release during the differentiation of M1 and M2 macrophages from 2 independent donors determined by ELISA on culture supernatants removed at the indicated time points. (I) Activin A levels released by M1 and M2 macrophages untreated (−) or stimulated with 10 or 100 ng/mL LPS for 24 hours, and either maintained in their conditioned medium (no wash) or in fresh culture medium (wash) during the LPS stimulation period. (J) Activin A levels released by murine M1 and M2 BMDM from 4 independent samples determined by ELISA. For panels G-J, the means and SD of triplicate determinations are shown; P < .005; **P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/19/10.1182_blood-2010-09-306993/4/m_zh89991170300001.jpeg?Expires=1767706305&Signature=qhLHhwB9JCQZEQK05RjO0mLjKjWfZ25CToy~VritN0uGQI66ixxAjryt1b6y~ut6HaYOcMVnac8UBBTy835JGml8OeiNUq9HygR1goW70D2yJFhmkJ-QuovdBptFAjHfwR17m9Q9kumSOKy2Ry-HwJoClYzM0XbVK-y-LCau~8B~cCMIS-6afcCacsTbx8SLS2BYhyYotTo7Wn0EhgPW4tR0~Ei4IKJIzo8I76X19dJVbBwKuRvnUNxBGq8t7mMAyfqDe4UGE7um1-84fce5wE7LHZlPm-yttcZrNwTof2T-9xur-JXsF25Tnhg1Rpl112v77kJJe501alQwB2eaoA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)