Abstract
CD41 expression is associated with the earliest stages of mouse hematopoiesis. It is notably expressed on some cells of the intra-aortic hematopoietic clusters, an area where the first adult-repopulating hematopoietic stem cells (HSCs) are generated. Although it is generally accepted that CD41 expression marks the onset of primitive/definitive hematopoiesis, there are few published data concerning its expression on HSCs. It is as yet uncertain whether HSCs express CD41 throughout development, and if so, to what level. We performed a complete in vivo transplantation analysis with yolk sac, aorta, placenta, and fetal liver cells, sorted based on CD41 expression level. Our data show that the earliest emerging HSCs in the aorta express CD41 in a time-dependent manner. In contrast, placenta and liver HSCs are CD41−. Thus, differential and temporal expression of CD41 by HSCs in the distinct hematopoietic territories suggests a developmental/dynamic regulation of this marker throughout development.
Introduction
CD41 (or integrin αIIb) is a marker of the megakaryocytic lineage and also of other clonogenic progenitors.1-5 CD41 marks the onset of murine primitive and definitive hematopoiesis.5-9 At embryonic day 7 (E7), yolk sac (YS) primitive erythroid progenitors express low levels of CD41.7 By E8.25/E9.5, most YS definitive hematopoietic progenitors express CD41 to high levels,7 and definitive hematopoietic progenitors in the intraembryonic para-aortic splanchnopleura or aorta-gonad-mesonephros (AGM) region also express CD41.5,7-9 These embryonic CD41+ hematopoietic cells possess no endothelial potential.10,11 Immunostainings show CD41+ hematopoietic cells within YS blood islands,7,8,12 intra-aortic clusters,12-14 and attached to vessel walls of the placenta labyrinth.15,16 Real-time vital imaging demonstrates that rare phenotypically defined hematopoietic stem cells (HSCs) become CD41+ as soon as they emerge from the aortic endothelium.13 Based on these findings, CD41 has been suggested as a marker expressed by nascent HSCs, denoting the onset of hematopoietic fate. However, it is still unclear whether HSCs express CD41 throughout development.
HSCs (as defined by the ability to long-term, high-level, multilineage repopulate the hematopoietic system of irradiated adult mouse recipients) start to be detected at E10.5 in the aorta and vitelline/umbilical arteries17-20 and emerge from hemogenic endothelium.13,21,22 HSCs are also in the YS, placenta, and fetal liver (FL) beginning at E11.5.23 Only few transplantation data are available for CD41-sorted AGM cells.24,25 When sorted in the context of other markers (CD48, CD150, CD45, and endomucin), HSCs were in both CD41+ and CD41− fractions. To clarify whether CD41 is a HSC marker during development, we performed a comprehensive analysis of CD41 expression (negative, intermediate, high) on HSCs (during and subsequent to the developmental time in which they are generated) in the various HSC-containing tissues. We show that all E11 AGM HSCs express CD41 to intermediate levels and that expression is time and hematopoietic territory dependent.
Methods
Embryos and cell preparations
Timed matings were set up between males of transgenic mouse line Ln7226 or Ly6A GFP17 and wild-type (C57BL/10 × CBA)F1 females; or C57BL6 Ly5.1 males and females. Vaginal plug day is E0. Recipients were (C57BL/10 × CBA)F1 or C57BL6 Ly5.2 (8-10 weeks old). Animals were housed according to institutional guidelines, and procedures were performed in compliance with Standards for Care and Use of Laboratory Animals with approval from the Erasmus MC ethical review board.
E11/E12 AGM and YS, and E12 placenta and E14 FL were dissected and collagenase dissociated (0.125% weight/volume, type 1, Sigma-Aldrich Chemie) or crushed (FL). Cells were washed, counted, and suspended in phosphate-buffered saline (PBS), 10% fetal calf serum, and penicillin/streptomycin. Cells were stained with antibodies (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) for 30 minutes on ice in PBS/fetal calf serum/penicillin/streptomycin, washed and stained with Hoechst 33258, before analysis or sorting on FACScan or ArialIII (BD Biosciences).
Hematopoietic assays
In vitro clonogenic analysis was performed on dilutions of sorted cells plated in methylcellulose (M3434; StemCell Technologies). Hematopoietic colonies were counted at day 12.
For in vivo HSC analysis, sorted cells (various cell doses) were intravenously coinjected with 2 × 105 wild-type spleen cells into irradiated (9 Gy split-dose, 137Cs-source) recipients. After 4 months, donor chimerism (Ln72 or Ly6A GFP) was analyzed by semiquantitative polymerase chain reaction (PCR). Signal quantitation was by DNA normalization (myogenin) and Ln72 or Ly6A GFP control DNA dilutions. For multilineage repopulation analysis, T, B, erythroid, and myeloid cells were sorted from recipient bone marrow (BM) and spleens after antibody staining (supplemental Table 1). Primary recipient BM (2 × 106) cells were injected into secondary irradiated recipients to assess self-renewal capacity. For the Ly5.2 recipients injected with Ly5.1 cells, percentage chimerism was determined by flow cytometry on blood after erythrocyte lysis (Beckman Coulter) and antibody and 7-amino-actinomycin D staining (supplemental Table 1).
Immunostaining
E11 Ly6A GFP embryos were fixed (2% paraformaldehyde/PBS, 4°C, 1-2 hours), cryoprotected (30% sucrose/PBS, 4°C overnight), Tissue Tek embedded, frozen (dry ice), and cryosectioned. Immunohistochemical staining was as described17 with anti-CD41 purified, anti–rat IgG1 biotin, and streptavidin-Cy5 (supplemental Table 1) and detected by laser scanning microscopy.
Results and discussion
Flow cytometric analysis of CD41 expression was performed on cells from E11 and E12 AGM and YS, E12 placenta, and E14 FL. Time points correspond to organ-specific peaks of HSC activity. In all tissues, AGM (Figure 1A,H), YS (Figure 1E), placenta (Figure 2A), and FL (Figure 2E), 3 distinct cell populations were observed: CD41−, CD41intermediate (int) and CD41high, in agreement with YS and AGM data.7 The highest percentage of CD41int + high cells is found in placenta, compared with AGM, YS, and FL. This is consistent with the high number of hematopoietic progenitors and HSCs in E12 placenta compared with the other tissues as previously described15,27,28 and the high proportion of megakaryocytic lineage (CD41high) cells as determined by coexpression of Gp1bβ (not shown). In all tissues, the frequency of CD41int cells is greater than that of CD41high cells.
Phenotypic and functional analyses of CD41-sorted cell fractions of E11 and E12 embryonic tissues. (A-D,J) E11 AGM, (H,I) E12 AGM, and (E-G) E11 YS. (A,E,H) Flow cytometric analysis of E11 AGM, E11 YS, and E12 AGM, respectively. Representative sorting gates (red represents CD41high; green, CD41int; and blue, CD41−). The percentage of cells in each fraction is indicated. (B,F) In vitro colony-forming unit in culture (CFU-C) analyses show the number of total hematopoietic progenitors per 1000 cells in each CD41-sorted fraction of E11 AGM and E11 YS cells. Each sample was analyzed in triplicate for each dilution. n = 4 for E11 AGM, and n = 2 for E11 YS. (C,G,I) In vivo hematopoietic repopulation analysis of CD41-sorted fractions of E11 AGM, E11 YS, and E12 AGM 4 months after transplantation. Percentage of repopulated mice showing greater than 10% donor chimerism in peripheral blood is shown. Numbers above columns indicate the number of mice repopulated/number of mice transplanted. Dose of injected cells is indicated as embryo equivalents (ee). n = 3 for E11 AGM, n = 4 for E11 YS, and n = 2 for E12 AGM. nd indicates not done. (D) CD41 immunostaining of E11 Ly6A GFP embryo section showing the ventral wall of the aorta. (Top panel) Red fluorescent CD41 expression in hematopoietic cells. (Middle panel) Green fluorescent Ly6A GFP expression in hematopoietic cells and some endothelial cells. (Bottom panel) Merged fluorescence. Yellow represents overlap of CD41 and Ly6A GFP expression in hematopoietic cells closely associated with the aortic endothelium. Image acquisition was from LSM510NLO/FCS confocal microscope (Carl Zeiss BV) with 40×/1.3 NA water objective and Vectashield medium (Vector Laboratories). LSM image software was used (Carl Ziess BV). (J) Representative semiquantitative PCR analysis of hematopoietic tissue DNA from (upper panel) a primary recipient injected with 3 ee of E11 AGM CD41int cells and (lower panel) peripheral blood DNA from 6 secondary recipients injected with BM cells from the primary recipient 4 months after transplantation. Donor indicates the human β-globin PCR fragment, and Myo indicates the myogenin DNA normalization control PCR fragment. DNA dilution controls (0%-100%) were used to quantitate percentages of donor chimerism that are indicated below each lane. PB indicates peripheral blood; Th, thymus; LN, lymph node; Sp, spleen; M, myeloid (sorted cells from BM); E, erythroid (sorted from BM); L, lymphoid (sorted from BM); B, B lymphoid (sorted from spleen); and T, T lymphoid (sorted from spleen).
Phenotypic and functional analyses of CD41-sorted cell fractions of E11 and E12 embryonic tissues. (A-D,J) E11 AGM, (H,I) E12 AGM, and (E-G) E11 YS. (A,E,H) Flow cytometric analysis of E11 AGM, E11 YS, and E12 AGM, respectively. Representative sorting gates (red represents CD41high; green, CD41int; and blue, CD41−). The percentage of cells in each fraction is indicated. (B,F) In vitro colony-forming unit in culture (CFU-C) analyses show the number of total hematopoietic progenitors per 1000 cells in each CD41-sorted fraction of E11 AGM and E11 YS cells. Each sample was analyzed in triplicate for each dilution. n = 4 for E11 AGM, and n = 2 for E11 YS. (C,G,I) In vivo hematopoietic repopulation analysis of CD41-sorted fractions of E11 AGM, E11 YS, and E12 AGM 4 months after transplantation. Percentage of repopulated mice showing greater than 10% donor chimerism in peripheral blood is shown. Numbers above columns indicate the number of mice repopulated/number of mice transplanted. Dose of injected cells is indicated as embryo equivalents (ee). n = 3 for E11 AGM, n = 4 for E11 YS, and n = 2 for E12 AGM. nd indicates not done. (D) CD41 immunostaining of E11 Ly6A GFP embryo section showing the ventral wall of the aorta. (Top panel) Red fluorescent CD41 expression in hematopoietic cells. (Middle panel) Green fluorescent Ly6A GFP expression in hematopoietic cells and some endothelial cells. (Bottom panel) Merged fluorescence. Yellow represents overlap of CD41 and Ly6A GFP expression in hematopoietic cells closely associated with the aortic endothelium. Image acquisition was from LSM510NLO/FCS confocal microscope (Carl Zeiss BV) with 40×/1.3 NA water objective and Vectashield medium (Vector Laboratories). LSM image software was used (Carl Ziess BV). (J) Representative semiquantitative PCR analysis of hematopoietic tissue DNA from (upper panel) a primary recipient injected with 3 ee of E11 AGM CD41int cells and (lower panel) peripheral blood DNA from 6 secondary recipients injected with BM cells from the primary recipient 4 months after transplantation. Donor indicates the human β-globin PCR fragment, and Myo indicates the myogenin DNA normalization control PCR fragment. DNA dilution controls (0%-100%) were used to quantitate percentages of donor chimerism that are indicated below each lane. PB indicates peripheral blood; Th, thymus; LN, lymph node; Sp, spleen; M, myeloid (sorted cells from BM); E, erythroid (sorted from BM); L, lymphoid (sorted from BM); B, B lymphoid (sorted from spleen); and T, T lymphoid (sorted from spleen).
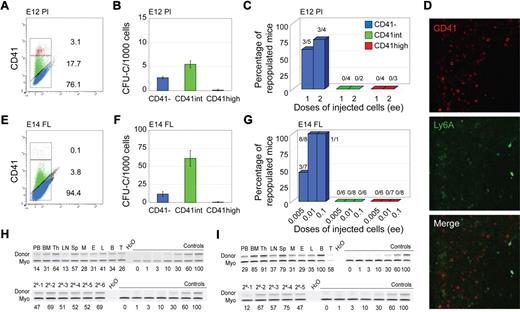
Phenotypic and functional analyses of CD41-sorted cell fractions of embryonic HSC reservoirs. E12 placenta (A-C,H) and E11/E14 liver (D-G,I). (A,E) Flow cytometric analysis of E12 placenta and E14 fetal liver (FL) showing representative sorting gates (red represents CD41high; green, CD41int; and blue, CD41−) and percentages of cells in each fraction. (B,F) In vitro colony-forming unit in culture (CFU-C) analyses showing the total number of hematopoietic progenitors per 1000 cells in each CD41-sorted fraction of E12 placenta and E14 FL cells. Each sample was analyzed in triplicate for each dilution. n = 2 for E12 placenta, and n = 3 for E14 FL. (C,G) In vivo hematopoietic repopulation analysis of CD41-sorted fractions of E12 placenta and E14 FL 4 months after transplantation. Percentage of repopulated mice showing greater than 10% donor chimerism in peripheral blood is shown. Numbers above columns indicate the number of mice repopulated/number of mice transplanted. Dose of injected cells is indicated as embryo equivalents (ee). n = 5 for E12 placenta, and n = 3 for E14 FL. (D) CD41 immunostaining of E11 Ly6A GFP embryo section showing the liver. (Top panel) Red fluorescent CD41 expression in hematopoietic cells. (Middle panel) Green fluorescent Ly6A GFP expression in hematopoietic cells. (Bottom panel) Merged fluorescence. The lack of yellow fluorescence indicates no coexpression of CD41 and Ly6A in liver hematopoietic cells. Image acquisition was from LSM510NLO/FCS confocal microscope (Carl Zeiss BV) with 40×/1.3 NA water objective and Vectashield medium (Vector Laboratories). LSM image software was used (Carl Ziess BV). (H-I) Representative semiquantitative PCR analysis of hematopoietic tissue DNA 4 months after transplantation from (H upper panel) a primary recipient injected with 2 ee of E12 placenta CD41− cells or (I upper panel) 0.1 ee of E14 FL. Representative semiquantitative PCR analysis of peripheral blood DNA from 6 secondary recipients injected with BM cells from the primary E12 placenta CD41− recipient (H lower panel) and 5 recipients injected with BM cells from the primary E14 FL CD41− recipient (I lower panel). Donor indicates the human β-globin PCR fragment, and Myo indicates the myogenin DNA normalization control PCR fragment. DNA dilution controls (0%-100%) were used to quantitate percentages of donor chimerism that are indicated below each lane. PB indicates peripheral blood; Th, thymus; LN, lymph node; Sp, spleen; M, myeloid (sorted from BM); E, erythroid (sorted from BM); L, lymphoid (sorted from BM); B, B lymphoid (sorted from spleen); and T, T lymphoid (sorted from spleen).
Phenotypic and functional analyses of CD41-sorted cell fractions of embryonic HSC reservoirs. E12 placenta (A-C,H) and E11/E14 liver (D-G,I). (A,E) Flow cytometric analysis of E12 placenta and E14 fetal liver (FL) showing representative sorting gates (red represents CD41high; green, CD41int; and blue, CD41−) and percentages of cells in each fraction. (B,F) In vitro colony-forming unit in culture (CFU-C) analyses showing the total number of hematopoietic progenitors per 1000 cells in each CD41-sorted fraction of E12 placenta and E14 FL cells. Each sample was analyzed in triplicate for each dilution. n = 2 for E12 placenta, and n = 3 for E14 FL. (C,G) In vivo hematopoietic repopulation analysis of CD41-sorted fractions of E12 placenta and E14 FL 4 months after transplantation. Percentage of repopulated mice showing greater than 10% donor chimerism in peripheral blood is shown. Numbers above columns indicate the number of mice repopulated/number of mice transplanted. Dose of injected cells is indicated as embryo equivalents (ee). n = 5 for E12 placenta, and n = 3 for E14 FL. (D) CD41 immunostaining of E11 Ly6A GFP embryo section showing the liver. (Top panel) Red fluorescent CD41 expression in hematopoietic cells. (Middle panel) Green fluorescent Ly6A GFP expression in hematopoietic cells. (Bottom panel) Merged fluorescence. The lack of yellow fluorescence indicates no coexpression of CD41 and Ly6A in liver hematopoietic cells. Image acquisition was from LSM510NLO/FCS confocal microscope (Carl Zeiss BV) with 40×/1.3 NA water objective and Vectashield medium (Vector Laboratories). LSM image software was used (Carl Ziess BV). (H-I) Representative semiquantitative PCR analysis of hematopoietic tissue DNA 4 months after transplantation from (H upper panel) a primary recipient injected with 2 ee of E12 placenta CD41− cells or (I upper panel) 0.1 ee of E14 FL. Representative semiquantitative PCR analysis of peripheral blood DNA from 6 secondary recipients injected with BM cells from the primary E12 placenta CD41− recipient (H lower panel) and 5 recipients injected with BM cells from the primary E14 FL CD41− recipient (I lower panel). Donor indicates the human β-globin PCR fragment, and Myo indicates the myogenin DNA normalization control PCR fragment. DNA dilution controls (0%-100%) were used to quantitate percentages of donor chimerism that are indicated below each lane. PB indicates peripheral blood; Th, thymus; LN, lymph node; Sp, spleen; M, myeloid (sorted from BM); E, erythroid (sorted from BM); L, lymphoid (sorted from BM); B, B lymphoid (sorted from spleen); and T, T lymphoid (sorted from spleen).
To test which CD41 fractions contain hematopoietic progenitors, sorted cells were plated in methylcellulose and colonies counted. Hematopoietic progenitors were found almost exclusively in the CD41int fraction of E11 AGM and YS cells (Figure 1B,F). Although the majority of E12 placenta and E14 FL hematopoietic progenitors were present in the CD41int fractions, some were also CD41− (Figure 2B,F). Together, these data suggest that CD41 expression on hematopoietic progenitors is developmentally regulated and/or dependent on the specific tissue microenvironment.
To examine whether HSCs during ontogeny express CD41, each sorted cell fraction from the distinct embryonic tissues was transplanted and donor cell engraftment examined at 4 months after transplantation. As expected, no mice were reconstituted with the CD41high fraction. E11 AGM HSCs were found exclusively in the CD41int fraction (Figure 1C) and were bona fide self-renewing HSCs as shown by high-level, multilineage engraftment of primary and secondary adult recipients (Figure 1J). CD41 expression colocalizes with expression of the Ly6A green fluorescent protein (GFP) HSC marker in some cells closely associated with the ventral aortic endothelium (Figure 1D), and multilineage self-renewing HSCs are enriched in the CD41intLy6A GFP+ fraction (supplemental Figure 1A-B). These data indicate, together with previous live imaging data,13 that CD41 is a marker for the earliest emerging aortic HSCs as they are transiting from endothelial to hematopoietic fate. In contrast, all E12 AGM HSCs were restricted to the CD41− fraction (Figure 1I), suggesting that CD41, as an AGM HSC marker, is developmentally time dependent. This is consistent with the data of McKinney-Freeman et al25 showing that, at E11.5, AGM HSCs are in both CD41+ and CD41− (CD150−CD48−) fractions.
CD41 expression on HSCs is not AGM restricted because, when sorted E11 (or E12, not shown) YS cells were transplanted, HSCs were found in both CD41− and CD41int fractions (Figure 1G). However, in E12 placenta (Figure 2C,H) and E14 FL (Figure 2G,I), HSCs were exclusively in the CD41− fraction. The presence of HSCs in the E12.5 placenta CD41+ fraction was previously reported, but, as stated by the authors, this was probably because of contaminating CD41− cells.25 At E11.5, no repopulation was found with either the CD41int or CD41− placenta cell fractions (n = 2) because of the extremely low HSC frequency at this time point in the placenta. Thus, we cannot conclude whether the first placental HSCs are CD41 expressing. Immunostainings of E11 FL sections (and flow cytometric analysis of E14 FL) show no overlap in expression of CD41 and Ly6A GFP (Figure 2D; supplemental Figure 1C-D). Thus, CD41 is expressed on HSCs in a time-dependent manner. CD41 expression now adds a further possibility for HSC enrichment during development when used in combination with other known HSC markers (Ly6A, c-kit, CD45, CD34, and CD150; supplemental Figures 1-2).
CD41 as a marker of the earliest HSCs generated in the AGM, together with the finding that some YS HSCs are CD41 expressing, may indicate HSC generation in the YS or HSC migration from the early AGM. CD41 expression on HSCs is lost with developmental time and HSC amplification in placenta, and FL is independent of CD41 expression. Hence, these data, showing that CD41 is a temporally restricted early HSC marker, are pivotal to future studies of its transcriptional regulation and role in HSC generation/migration.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Henk Dronk and Experimental Dieren Centrum (Erasmus MC) for mouse care and Reinier van der Linden and Fredrik Wallberg for cell sorting.
This work was supported by The Netherlands Organisation for Scientific Research (VIDI grant 917-76-345, C.R.; and VICI grant 916-36-601, E.D.) and the National Institutes of Health (Merit Award R37 DK51077, E.D.).
National Institutes of Health
Authorship
Contribution: C.R. performed experiments and wrote the paper; K.O., J.-C.B., and A.O. performed experiments; and E.D. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation of K.O. is Department of Haematology, Cambridge Institute for Medical Research, University of Cambridge, Cambridge, United Kingdom.
Correspondence: Elaine Dzierzak, Erasmus Medical Center, Erasmus MC Stem Cell Institute, Department of Cell Biology, PO Box 2040, 3000 CA Rotterdam, The Netherlands; e-mail: e.dzierzak@erasmusmc.nl.
References
Author notes
C.R. and K.O. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal