Abstract
It is not known whether pandemic 2009 influenza A/H1N1 (2009 H1N1) leads to more serious disease than seasonal influenza in hematopoietic cell transplant (HCT) recipients. In a retrospective study in HCT recipients with virologically proven influenza virus infection, a total of 161 HCT recipients (18 2009 H1N1, 103 seasonal influenza A, and 40 seasonal influenza B) were analyzed. In multivariable analyses, more patients with 2009 H1N1 had lower respiratory tract disease (LRD), hypoxemia, and prolonged viral shedding compared with seasonal influenza A. Seasonal influenza A and B outcomes were similar. There was no difference in overall and influenza-associated mortality among influenza virus types. Both early and delayed administration of antiviral therapy was shown to be beneficial in terms of decreased rates of development of LRD, although earlier intervention appeared to be more effective. Profound lymphopenia and lack of early antiviral therapy were associated significantly with LRD, hypoxemia, and death. High-dose corticosteroid treatment (≥ 1 mg/kg) given at the time of influenza diagnosis was associated with a reduced risk for mechanical ventilation. Thus, our data suggest that infection with 2009 influenza A/H1N1 resulted in more severe respiratory disease in HCT recipients compared with seasonal influenza.
Introduction
The epidemiology of influenza virus infection in hematopoietic cell transplant (HCT) recipients closely parallels the occurrence of influenza infection in the community. However, the clinical presentation, severity of disease, and outcomes are quite different.1-5 HCT recipients with symptomatic upper respiratory tract infection have a greater tendency to progress to severe pneumonia, and the development of pneumonia is closely linked to influenza-related mortality. Previous studies revealed that lymphopenia, steroid use, and antiviral therapy may affect the risk of pneumonia.3,6,7 In addition to these host factors, the type and subtype of influenza viruses may also impact the severity and outcome of illness. In immunocompetent adults, influenza B virus is known to cause less severe disease than influenza A virus.8 Disease severity may vary among different influenza A virus subtypes, with the highest rates of pneumonia and mortality documented after avian influenza A/H5N1, with mortality rates that approach 60%.9
The recent pandemic caused by novel 2009 influenza A/H1N1 (2009 H1N1) impacted persons of all ages and diverse underlying health conditions. Animal studies and pathologic observations suggest that 2009 H1N1 can cause more severe respiratory tract disease than seasonal influenza viruses.10-13 However, the severity of diseases and clinical outcomes according to influenza type or subtype, especially differences between 2009 H1N1 and seasonal influenza virus infection, have not been studied in HCT recipients. We conducted a retrospective study in HCT recipients with laboratory-confirmed influenza virus infection to define the impact of influenza virus subtype on the severity of disease and clinical outcomes.
Methods
Patients
We reviewed virology and clinical records from January 1991 through November 2009 and identified all transplantation recipients who were diagnosed with influenza infection based on virologic testing. Only the first episode of documented influenza infection in each patient was analyzed. Selected aspects of seasonal influenza A and B and selected seasonal H1N1 cases have been reported earlier.3,14-17 Each patient had previously signed an informed consent form that allowed clinical information for future research, in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center.
Virologic procedures and clinical management
A targeted surveillance strategy was used throughout the study period. A nasopharyngeal-throat wash was performed in patients with upper respiratory tract symptoms, and bronchoalveolar lavage was the standard of care in patients with new pulmonary infiltrates. Viral culture and direct fluorescent antibody staining for influenza A and B were performed on all respiratory specimens. Since March 2006, reverse-transcriptase polymerase chain reaction (RT-PCR) was performed in addition to these tests, as previously described.18 Nasopharyngeal-throat washes were generally obtained weekly until clearance of the infection. Antiviral therapy was prescribed at the discretion of the treating physician, based on availability of drug and general seasonal treatment recommendations. M2 inhibitors for influenza A were initially used. Oseltamivir was administered once available, generally at a dose of 75 mg twice daily for upper respiratory tract disease (URD) and 150 mg twice daily for lower respiratory tract disease (LRD).5
Criteria for analysis and definitions
URD was defined as influenza documented in an upper respiratory tract specimen in a patient with compatible symptoms in the absence of radiographic or clinical evidence of pneumonia. LRD was defined as a new pulmonary infiltrate in association with a positive lower respiratory tract specimen or a positive upper respiratory tract specimen for patients who did not undergo bronchoalveolar lavage. Hypoxemia was defined as ambient air oxygen saturation less than 90% or the need for O2 supplementation, and mechanical ventilation was defined as any mechanical ventilation assistance, such as continuous positive airways pressure, bilevel positive airway pressure, and intubation, occurring during the 28 days after a positive influenza specimen. Death was considered to be associated with influenza if a patient died of respiratory failure and influenza virus was considered a contributor to the lung injury. Prolonged shedding was defined as viral excretion lasting longer than 14 days.
The lowest absolute lymphocyte count encountered during the 2 weeks before influenza diagnosis was used to determine lymphopenia. For the analysis of corticosteroid therapy, patients were classified in 3 different groups depending on the dosage of corticosteroids taken during the 2 weeks before influenza diagnosis. The first group consisted of patients who received no corticosteroids, and the second group (“low-dose group”) consisted of patients who received prednisone/methylprednisolone at a dosage of less than 1 mg/kg per day or beclomethasone dipropionate at any dosage. Oral beclomethasone dipropionate is used to treat gastrointestinal graft-versus-host disease (GVHD) and has an active metabolite that reaches pulmonary circulation and may prevent noninfectious pulmonary complications after HCT.19-21 The third group (“high-dose group”) consisted of patients who received 1 mg/kg per day or more of prednisone/methylprednisolone. Dexamethasone doses were converted to a prednisone equivalent dose for analysis. Corticosteroid dose category was assigned according to the highest daily dose taken by the patient during the 2 weeks preceding influenza diagnosis.
Initiation of antiviral therapy for URD was based on the time between influenza URD diagnosis and the initiation of antiviral treatment and categorized into 3 groups; the first group consisted of patients who received any appropriate antiviral therapy (rimantadine, amantadine, oseltamivir, or zanamivir) within 48 hours after URD diagnosis. The second group included patients who received antiviral therapy after 48 hours after URD diagnosis, and the untreated group included patients with no antiviral therapy ever after URD diagnosis and with LRD at the presentation. Underlying disease risk was categorized as advanced and nonadvanced according to disease status at time of HCT using previously published criteria.22 Combination therapy was given to some patients with LRD at the discretion of the treating physician.
Statistical analysis
All statistical analyses were performed with SAS Version 9.1 (SAS Institute). χ2 test or the Fisher exact test was used for the analysis of categorical variables, and Student t test or the Wilcoxon rank-sum test was used for continuous variables between 2 groups, and analysis of variance or Kruskal-Wallis test were used for comparison of continuous variables among 3 groups. To define the severity of disease and clinical outcome, analyses were done for the following 6 endpoints: (1) LRD, (2) hypoxemia, (3) mechanical ventilation, (4) time to death of all causes, (5) time to influenza-associated death, and (6) prolonged shedding. Univariable and multivariable logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for LRD, hypoxemia, mechanical ventilation, and prolonged shedding. The probability of survival was estimated by the Kaplan-Meier method, and univariable hazards for mortality were compared using log-rank tests. Multivariable Cox proportional hazards models were used to evaluate adjusted hazard ratios (HRs) for mortality.
Covariates evaluated as candidates for inclusion in multivariable models included age, sex, underlying disease, disease risk at transplantation, donor type, human leukocyte antigen match, stem cell source, intensity of the conditioning regimen, acute GVHD, lymphocyte count, corticosteroid treatment, early antiviral therapy, year of influenza diagnosis, and influenza virus type. Variables with P value < .3 in the univariable models were considered as possible predictor variables and were retained for the multivariable models. Corticosteroid treatment, early antiviral therapy for upper respiratory tract infection, lymphocyte count, and year of influenza diagnosis were forced into the multivariable models for all endpoint analyses. Two-sided P values < .05 were considered statistically significant.
Results
Patient characteristics
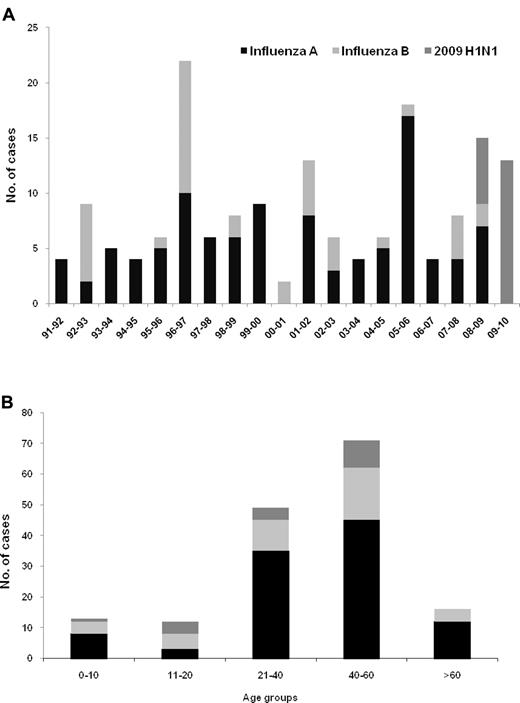
During the study period, 165 HCT recipients with virologically proven influenza virus respiratory tract disease were identified (one patient had 2 episodes). Three asymptomatic patients with 2009 H1N1 detected during an active weekly surveillance study for respiratory virus infection and one who received oseltamivir as a postexposure prophylaxis after close contact with 2009 H1N1 patient were excluded (one of the 4 died of relapse of acute leukemia 102 days after the detection of 2009 H1N1 virus; 2 received antiviral treatment until the last contact at outpatient clinic [median, 71 days; range, 14-219 days]). As a result, 161 HCT recipients were included for analyses. The types of influenza virus throughout the study period and by age groups are presented in Figure 1. Influenza was documented in a median of 6 patients (range, 2-22 patients) per season with a predominance of influenza A (5:1, A vs B). Overall, 103 cases of seasonal influenza A, 40 cases of seasonal influenza B, and 18 of 2009 H1N1 were included. Three of 18 patients with 2009 H1N1 were confirmed as influenza A but not subtyped as pandemic H1N1 during the peak phase of the pandemic; these were included as H1N1 for this analysis. Six patients with 2009 H1N1 diagnosed during May and June were included in 2008 to 2009 season.
Type of influenza virus among 161 HCT recipients. (A) By 2-year period of transplantation. (B) By age. One subject had 2 episodes (seasonal A in 2005 and A/H1N1 in 2009, panel A).
Type of influenza virus among 161 HCT recipients. (A) By 2-year period of transplantation. (B) By age. One subject had 2 episodes (seasonal A in 2005 and A/H1N1 in 2009, panel A).
Characteristics of patients are listed in Table 1. There was no difference in the proportion of age, sex, underlying diseases (data not shown), underlying disease risk at transplantation, transplantation type, stem cell source, and acute GVHD (data not shown) among the 3 groups infected with seasonal influenza A, B, or 2009 H1N1. However, patients infected with 2009 H1N1 acquired their influenza infection later after transplantation (P = .018, Table 1), and there were differences in the distribution of lymphocyte count and corticosteroid treatment among the 3 groups (P = .021 and P = .006, respectively, Table 1).
Characteristics of HCT recipients with influenza virus respiratory tract disease
| Characteristics . | Virus type . | All patients (N = 161) . | ||
|---|---|---|---|---|
| Influenza A (N = 103) . | Influenza B (N = 40) . | 2009 H1N1 (N = 18) . | ||
| Age, y, median (IQR) | 42 (32-54) | 41.5 (24-51) | 41.5 (18-52) | 42 (30-52) |
| Time after transplant, d, median (IQR) | 78 (40-197) | 69 (41-212) | 601.5 (270-977) | 80 (44-294) |
| Male sex | 62 (60.2) | 21 (52.5) | 8 (44.4) | 91 (56.5) |
| Disease risk at transplantation | ||||
| Nonadvanced | 59 (57.3) | 19 (47.5) | 9 (50.0) | 87 (54.0) |
| Advanced | 44 (42.7) | 21 (52.5) | 9 (50.0) | 74 (46.0) |
| Transplant type | ||||
| Autologous | 15 (14.6) | 11 (27.5) | 5 (27.8) | 31 (19.3) |
| Matched related | 39 (37.9) | 16 (40.0) | 4 (22.2) | 59 (36.6) |
| Mismatched or unrelated | 49 (47.6) | 13 (32.5) | 9 (50.0) | 71 (44.1) |
| Stem cell source | ||||
| Bone marrow or UBC | 45 (43.7%) | 20 (50.0%) | 5 (27.8%) | 70 (43.5%) |
| Peripheral blood | 58 (56.3%) | 20 (50.0%) | 13 (72.2%) | 91 (56.5%) |
| Conditioning regimen | ||||
| Myeloablative | 84 (81.4%) | 34 (85.0%) | 15 (83.3%) | 133 (82.6%) |
| Reduced intensity | 19 (18.5%) | 6 (15.0%) | 3 (16.7%) | 28 (17.4%) |
| Lymphocyte count (cells/mm3)* | ||||
| < 100 | 20 (20.0) | 18 (45.0) | 5 (29.4) | 43 (27.4) |
| 100-300 | 35 (35.0) | 6 (15.0) | 3 (17.6) | 44 (20.0) |
| > 300 | 45 (45.0) | 16 (40.0) | 9 (52.9) | 70 (44.6) |
| Corticosteroid treatment† | ||||
| None | 43 (41.7) | 20 (50.0) | 7 (38.9) | 70 (43.5) |
| Low-dose | 27 (26.2) | 16 (40.0) | 10 (55.6) | 53 (32.9) |
| High-dose | 33 (32.0) | 4 (10.0) | 1 (15.0) | 38 (23.6) |
| Early antiviral therapy for URD | ||||
| Yes (≤ 48 h)‡ | 39 (38.2) | 6 (15.0) | 7 (38.9) | 52 (32.5) |
| No (> 48 h)‡ | 11 (10.8) | 2 (5.0) | 6 (33.3) | 19 (11.9) |
| No antiviral therapy for URD§ | 52 (51.0) | 32 (80.0)‖ | 5 (27.8) | 89 (55.6) |
| Characteristics . | Virus type . | All patients (N = 161) . | ||
|---|---|---|---|---|
| Influenza A (N = 103) . | Influenza B (N = 40) . | 2009 H1N1 (N = 18) . | ||
| Age, y, median (IQR) | 42 (32-54) | 41.5 (24-51) | 41.5 (18-52) | 42 (30-52) |
| Time after transplant, d, median (IQR) | 78 (40-197) | 69 (41-212) | 601.5 (270-977) | 80 (44-294) |
| Male sex | 62 (60.2) | 21 (52.5) | 8 (44.4) | 91 (56.5) |
| Disease risk at transplantation | ||||
| Nonadvanced | 59 (57.3) | 19 (47.5) | 9 (50.0) | 87 (54.0) |
| Advanced | 44 (42.7) | 21 (52.5) | 9 (50.0) | 74 (46.0) |
| Transplant type | ||||
| Autologous | 15 (14.6) | 11 (27.5) | 5 (27.8) | 31 (19.3) |
| Matched related | 39 (37.9) | 16 (40.0) | 4 (22.2) | 59 (36.6) |
| Mismatched or unrelated | 49 (47.6) | 13 (32.5) | 9 (50.0) | 71 (44.1) |
| Stem cell source | ||||
| Bone marrow or UBC | 45 (43.7%) | 20 (50.0%) | 5 (27.8%) | 70 (43.5%) |
| Peripheral blood | 58 (56.3%) | 20 (50.0%) | 13 (72.2%) | 91 (56.5%) |
| Conditioning regimen | ||||
| Myeloablative | 84 (81.4%) | 34 (85.0%) | 15 (83.3%) | 133 (82.6%) |
| Reduced intensity | 19 (18.5%) | 6 (15.0%) | 3 (16.7%) | 28 (17.4%) |
| Lymphocyte count (cells/mm3)* | ||||
| < 100 | 20 (20.0) | 18 (45.0) | 5 (29.4) | 43 (27.4) |
| 100-300 | 35 (35.0) | 6 (15.0) | 3 (17.6) | 44 (20.0) |
| > 300 | 45 (45.0) | 16 (40.0) | 9 (52.9) | 70 (44.6) |
| Corticosteroid treatment† | ||||
| None | 43 (41.7) | 20 (50.0) | 7 (38.9) | 70 (43.5) |
| Low-dose | 27 (26.2) | 16 (40.0) | 10 (55.6) | 53 (32.9) |
| High-dose | 33 (32.0) | 4 (10.0) | 1 (15.0) | 38 (23.6) |
| Early antiviral therapy for URD | ||||
| Yes (≤ 48 h)‡ | 39 (38.2) | 6 (15.0) | 7 (38.9) | 52 (32.5) |
| No (> 48 h)‡ | 11 (10.8) | 2 (5.0) | 6 (33.3) | 19 (11.9) |
| No antiviral therapy for URD§ | 52 (51.0) | 32 (80.0)‖ | 5 (27.8) | 89 (55.6) |
Data are no. (%) of patients, unless otherwise indicated. Influenza A and B indicate seasonal influenza in all the tables and figures. One patient had H1N1 several years after experiencing an infection; with seasonal influenza A, only the first episode was included in the analyses.
IQR indicates interquartile range; MDS, myelodysplastic syndrome; and UBC, umbilical cord blood.
Lymphocyte counts were not available in 4 patients.
Low-dose indicates < 1 mg/kg of prednisone or oral beclomethasone diproprionate; and High-dose, ≥ 1 mg/kg of prednisone or prednisone equivalent dose of other steroids.
Time between influenza URD diagnosis and initiation of antiviral treatment.
This includes patients who receive no antiviral treatment ever after influenza diagnosis and who had LRD at the presentation.
Eighteen of 32 were diagnosed as influenza B infection before the regulatory approval of the neuraminidase inhibitors.
Antiviral treatment for influenza was administered in 102 (63.4%) patients (17 [94.4%] in 2009 H1N1 vs 85 [59.4%] in seasonal influenza, P = .003). Twenty-one of 102 patients were treated with 2 or more antiviral drugs (6 of 17 [35.3%] in 2009 H1N1 vs 15 of 85 [17.6%] in seasonal influenza, P = .1). Precise antiviral therapy history for starting time was available in 160 (99.4%) patients. Early antiviral therapy within 48 hours after URD diagnosis was achieved in 7 (38.9%) patients with 2009 H1N1, 39 (38.2%) with seasonal influenza A, and 6 (15.0%) with seasonal influenza B (P < .001; Table 1). Eighteen patients who had influenza B infection and did not receive antiviral therapy were diagnosed before the regulatory approval of the neuraminidase inhibitors (Table 1).
Clinical outcomes of influenza virus infection among HCT recipients
Clinical outcomes according to influenza virus are listed in Table 2. Overall, 42 (26.1%) patients developed LRD, 43 (26.7%) had hypoxemia, and 17 (10.6%) required mechanical ventilation. Twenty (12.4%) patients died within 42 days after the diagnosis of influenza (11 [10.7%] seasonal influenza A, 6 [15.0%] influenza B, and 3 [16.7%] 2009 H1N1, P = .59). Among these, influenza-associated deaths occurred in a total of 15 (9.3%) patients (8 [7.8%] seasonal influenza A, 4 [10.0%] influenza B, and 3 [16.7%] 2009 H1N1, P = .43). Among 42 patients who developed LRD, 17 (40.5%) required mechanical ventilation and 15 (35.7%) died as a consequence of influenza infection.
Impacts of influenza virus type on clinical outcomes among HCT recipients, by multivariable analyses
| Outcome . | Virus type . | Influenza B* . | 2009 H1N1* . | ||||
|---|---|---|---|---|---|---|---|
| Influenza A (N = 103) . | Influenza B (N = 40) . | 2009 H1N1 (N = 18) . | Adjusted OR or HR (95% CI)† . | P . | Adjusted OR or HR (95% CI)† . | P . | |
| LRD | 22 (21.4) | 10 (25.0) | 10 (55.6) | 0.70 (0.3-1.9) | .49 | 6.48 (1.4-31) | .02 |
| Hypoxemia | 22 (21.4) | 11 (27.5) | 10 (55.6) | 0.85 (0.3-2.1) | .73 | 3.96 (1.1-15) | .04 |
| Mechanical ventilation | 8 (7.8) | 4 (10.0) | 5 (27.8) | 0.39 (0.1-1.8) | .24 | 2.59 (0.5-15) | .29 |
| Time to death by day 42 | 11 (10.7) | 6 (15.0) | 3 (16.7) | 0.95 (0.3-2.7) | .92 | 0.93 (0.2-4.6) | .93 |
| Time to influenza-associated death | 8 (7.8) | 4 (10.0) | 3 (16.7) | 0.86 (0.2-3.0) | .82 | 1.15 (0.2-5.9) | .87 |
| Outcome . | Virus type . | Influenza B* . | 2009 H1N1* . | ||||
|---|---|---|---|---|---|---|---|
| Influenza A (N = 103) . | Influenza B (N = 40) . | 2009 H1N1 (N = 18) . | Adjusted OR or HR (95% CI)† . | P . | Adjusted OR or HR (95% CI)† . | P . | |
| LRD | 22 (21.4) | 10 (25.0) | 10 (55.6) | 0.70 (0.3-1.9) | .49 | 6.48 (1.4-31) | .02 |
| Hypoxemia | 22 (21.4) | 11 (27.5) | 10 (55.6) | 0.85 (0.3-2.1) | .73 | 3.96 (1.1-15) | .04 |
| Mechanical ventilation | 8 (7.8) | 4 (10.0) | 5 (27.8) | 0.39 (0.1-1.8) | .24 | 2.59 (0.5-15) | .29 |
| Time to death by day 42 | 11 (10.7) | 6 (15.0) | 3 (16.7) | 0.95 (0.3-2.7) | .92 | 0.93 (0.2-4.6) | .93 |
| Time to influenza-associated death | 8 (7.8) | 4 (10.0) | 3 (16.7) | 0.86 (0.2-3.0) | .82 | 1.15 (0.2-5.9) | .87 |
Data are no. (%) of patients, unless otherwise indicated. The year of influenza diagnosed was forced into the multivariable models for all endpoint analyses. Influenza virus type, lymphocyte count, and early antiviral therapy were included as variables in all analyses. Multivariable models also included stem cell source for LRD analysis, acute GVHD for hypoxemia analysis, and corticosteroid treatment for mechanical ventilation analysis.
Seasonal influenza A is the reference.
ORs are shown for risk for LRD, hypoxemia, and mechanical ventilation, and HRs are shown elsewhere.
A total of 102 (63.0%) patients had 2 or more upper respiratory tract specimens sampled that permitted viral clearance analysis. Median duration of shedding was 7.5 days (range, 2.5-80.5 days) and prolonged shedding over 14 days was observed in 33 (32.4%) of patients in overall (19 [30.2%] seasonal influenza A, 8 [28.6%] influenza B, and 6 [54.5%] 2009 H1N1, P = .26). Median duration of shedding was 27 days in patients with 2009 H1N1 and 7 days in patients with seasonal influenza.
Impact of influenza virus type on clinical outcomes
In univariable analyses with seasonal influenza A as reference, significantly worse outcomes were detected for 2009 H1N1 with regard to LRD (OR, 4.60; 95% CI, 1.6-13; P = .004), hypoxemia (OR, 4.60; 95% CI, 1.6-13; P = .004), and mechanical ventilation (OR, 4.57; 95% CI, 1.3-16; P = .018); there were no differences in survival according to influenza types.
Multivariable analyses were carried out for each clinical outcome studied (Table 2), and year of influenza diagnosis was forced into the multivariable models for all endpoint analyses to account for possible changes in supportive care over time. As with univariable analyses, 2009 H1N1 influenza virus was associated with a significantly increased risk of LRD and hypoxemia compared with seasonal influenza A; there was no statistically significant difference in clinical outcomes between seasonal influenza A and B. In addition, patients with 2009 H1N1 required mechanical ventilatory support more frequently, although these differences did not reach statistical significance. Influenza virus type was not associated with time to death by multivariable Cox proportional hazards models (Table 2).
Significance of lymphopenia
Multivariable analyses demonstrated that profound lymphopenia (< 100 cells/μL) compared with lymphocyte count more than 300 cells/μL was a significant risk factor for LRD (OR, 5.44; 95% CI, 1.7-18; P = .004), hypoxemia (OR, 3.12; 95% CI, 1.2-8.2; P = .021), mechanical ventilation (OR, 13.3; 95% CI, 2.8-63; P = .001), time to overall death by day 42 after influenza diagnosis (HR, 4.04; 95% CI, 1.2-13; P = .021), and time to influenza-associated death (HR, 7.34; 95% CI, 1.5-35; P = .012).
Timing of antiviral treatment
Early antiviral therapy for URD within the first 48 hours after influenza diagnosis was associated with a significant risk reduction for LRD (adjusted OR, 0.04; 95% CI, 0.0-0.2; P < .001) and hypoxemia (adjusted OR, 0.14; 95% CI, 0.0-0.4; P < .001); it also reduced overall death at 6 weeks (adjusted HR, 0.21; 95% CI, 0.0-1.0; P = .049) and 6 months (adjusted HR, 0.3; 95% CI, 0.1-0.8; P = .014). In addition, early therapy showed a trend toward reduced risk for influenza-associated death (adjusted HR, 0.15; 95% CI, 0.0-1.3; P = .086) compared with no antiviral therapy for upper respiratory tract infection. Antiviral therapy given after 48 hours after URD diagnosis was still associated with a significant risk reduction for LRD (adjusted OR, 0.14; 95% CI, 0.0-0.7; P = .019).
Impact of corticosteroids
Patients who received high-dose corticosteroid treatment (≥ 1 mg/kg) had an inverse association with mechanical ventilation compared with patients who did not receive steroid treatment (adjusted OR, 0.07; 95% CI, 0.0-0.7; P = .025). A univariable subgroup analysis according to influenza types was performed to determine the impact of steroid treatment on mechanical ventilation. Among patients with seasonal influenza A (n = 103), mechanical ventilation was required in 7 (16.3%) of 43 patients who did not receive steroid treatment compared with only 1 (3.0%) of 33 patients with high-dose steroid treatment (P = .03). Similar trends were seen for both influenza B and 2009 H1N1 with cases of mechanical ventilation in recipients of high-dose steroids, but the associations were not statistically significant (although sample size was small). Because autologous transplant recipients did not receive high-dose steroids, a stratified multivariable analysis was performed for mechanical ventilation in autologous and allogeneic transplant recipients. No effect of low-dose steroid treatment was seen in autologous recipients; the protective effect of high-dose steroids was confirmed in allogeneic transplant recipients (data not shown).
Viral shedding
The 2009 H1N1 was a significant risk factor for prolonged viral shedding compared with seasonal influenza A (adjusted OR, 5.90; 95% CI, 1.1-33; P = .041). Patients treated with high-dose corticosteroid showed a trend toward prolonged shedding compared with patients without steroid treatment (adjusted OR, 2.81; 95% CI, 0.9-9.2; P = .09; 23.6% in high-dose steroids, 32.9% in low-dose steroids, 43.5% in no steroids). In addition, HCT using bone marrow and cord blood as cell source was a significant risk for prolonged viral shedding compared with peripheral blood stem cell (adjusted OR, 3.78; 95% CI, 1.2-12; P = .024).
Discussion
This study demonstrates that the influenza virus type and subtype affect the severity of disease and clinical outcomes in HCT recipients. Infection with 2009 H1N1 was significantly associated with the development of LRD and hypoxemia and showed a trend toward more mechanical ventilation than infection with seasonal influenza A. However, infection with 2009 H1N1 was not associated with increased overall mortality compared with either influenza B or other influenza A subtypes. By contrast, there were no apparent differences in outcomes between seasonal influenza A and B. We also demonstrate that early antiviral therapy was associated with a statistically significant benefit with regard to clinical outcomes, including development of LRD, hypoxemia, and death. Importantly, even a later start of antiviral drugs (> 48 hours after virologic diagnosis) was associated with a reduced risk of LRD. Finally, our data demonstrate that acute lung injury requiring mechanical ventilation was less frequent in patients who received high-dose corticosteroids for the treatment of GVHD than in those who did not.
Previous studies of seasonal influenza in HCT recipients have consistently demonstrated a higher rate of progression to LRD and need for mechanical ventilatory support,6 prolonged viral shedding,23 and a higher mortality3,24-26 in HCT recipients than in the general population. Although rates of infection and hospitalization after 2009 influenza H1N1 are remarkably different by age group from rates reported in previous years,27 clinical disease in the general population after infection with 2009 H1N1 and seasonal influenza have been shown to be remarkably similar with regard to overall disease severity and rates of complications.28 To our knowledge, our study is the first to directly compare the severity of disease and the differences in clinical outcome among HCT recipients infected with 2009 H1N1 and seasonal influenza A and B virus infection. In contrast to the findings in the general population,24 our study demonstrated more severe clinical sequelae after infection with 2009 H1N1 than after other infection with other seasonal influenza A and B viruses. Similarly, Strouse et al reported that 2009 H1N1 caused more frequently severe illness in patients with sickle cell disease compared with seasonal influenza.29 It is possible that the 2009 H1N1 strain may cause more serious disease than seasonal influenza because of little or no preexisting immunity to the new strain in the stem cell donor or, in the nonmyeloablative setting, also in the recipient. In animal studies, the level of pulmonary replication of the 2009 H1N1 virus has been reported to be higher than that of seasonal influenza A/H1N1 viruses.11-13 It has also been reported that infection with 2009 H1N1 virus resulted in similar pathology to that encountered with highly virulent influenza A viruses, such as the 1918 H1N1 and H5N1 viruses.10
The characteristics of 2009 H1N1 in HCT recipients are only slowly emerging. Redelman-Sidi et al reported 21 HCT recipients with 2009 H1N1, of whom 40% had LRD and 37% required hospitalization; no cases of respiratory failure or death were attributed to influenza infection in this study.30 George et al reported LRD and hospitalization in 5 of 13 cases; 4 required mechanical ventilation and eventually died during that admission.31 Taplitz et al showed that 52% of 27 patients with 2009 H1N1 had LRD, half of them required mechanical ventilation, and the overall influenza-associated mortality was 22%.7 The reported number of patients in each of these and other recent reports32-34 is relatively small, and the clinical outcomes of 2009 H1N1 vary somewhat differently in the different reports. Moreover, the disease severity of 2009 H1N1 compared with seasonal influenza was not described. In our cohort, the proportion of LRD, hypoxemia, mechanical ventilation, and deaths in HCT patients with 2009 H1N1 was higher than those reported by Redelman-Sidi et al30 and Cost et al35 as well as those in solid-organ transplant recipients,36 but consistent with the recent report by Taplitz et al.7
At the beginning of the pandemic, many experts, agencies, and transplant societies recommended that transplant recipients with pandemic H1N1 should be started on antiviral treatment regardless of symptom duration or even definitive diagnosis, and that combination antiviral therapy and high-dose oseltamivir could be considered for LRD.5,37 We used a higher dose of oseltamivir (150 mg, twice daily) because of the questionable absorption in patients with GVHD-associated malabsorption, as well as the higher viral load and prolonged shedding in HCT recipients. In addition, combination therapy, which was based on in vitro and animal data38-40 and resulting from emergence of resistance,41 was administered more frequently at our institution in patients with 2009 H1N1 than in patients with seasonal influenza. In our study, antiviral therapy given within 48 hours after the sample for virologic diagnosis was obtained reduced LRD, hypoxemia, overall death, and influenza-associated death. A new finding is that antiviral therapy initiated after 48 hours of URD diagnosis still had clinical benefit. This extends and adds statistical power to our recent analysis on the timing of antiviral treatment15 and is consistent with recent data for H5N1 influenza.42
Viral shedding was affected by stem cell source and virus type. We speculate that the prolonged shedding in patients undergoing marrow and cord blood transplantation may be the result of impaired cell-mediated immunity. The observed effect of 2009 H1N1 on shedding may be, at least in part, explained by the preferential use of PCR testing (and its higher sensitivity) for 2009 H1N1. The association of high-dose steroids with prolonged viral shedding did not reach statistical significance in the multivariable analysis. However, there was a protective effect of corticosteroids on subsequent requirements for mechanical ventilation. An earlier study from our center indicated no adverse outcomes of high-dose steroids, but there was also no benefit for any of the outcomes, although statistical trends were observed.15 The increased sample size of the present study added statistical power and provided more robust results. The protective effect of steroids was present for all viral subtypes. This is an important finding because of the concern of clinicians to prescribe corticosteroid therapy during acute viral infections, and the general practice to reduce steroid doses because of concerns of adverse effects on viral replication. Our study challenges these practices, but confirmatory studies are needed to validate our findings in different cohorts and, ultimately, in randomized trials. Support for this strategy comes from data on the potentially harmful effect of the influenza-induced inflammatory cytokine responses43-45 as well as from a recent study that reported that prolonged corticosteroid therapy could be combined with oseltamivir to treat acute lung injury in patients with or without confirmed 2009 H1N1 infection.46
This study is strengthened because of the consistent targeted surveillance strategy in place throughout the study period, which minimized the possibility that patients likely to come to medical attention were those with more severe disease. In addition, we attempted to overcome other biases by applying multivariable analyses and by directly comparing outcomes with seasonal influenza. Our study evaluated the largest number of HCT recipients with laboratory confirmed influenza virus infection to date and directly compared outcomes by virus subtype in multivariable models. With this approach, we were able to analyze the timing of antiviral drug treatment and corticosteroid dosing regimens by accounting for confounders that could otherwise influence the results. However, several limitations should be noted. The number of patients infected with 2009 H1N1 was relatively small, limiting our ability to conclusively determine the true risk of mechanical ventilation and attributable mortality of 2009 H1N1 compared with seasonal influenza. Combined datasets from several institutions will be needed to analyze these endpoints with more statistical power. In addition, we had no subtype data of seasonal influenza A virus, and precise data on the type of clinical symptoms at presentation, onset of symptoms, and vaccination history were not available because of the retrospective nature of our study. Nevertheless, we found that HCT recipients with 2009 H1N1 often presented with nonspecific and atypical symptoms (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) as previously reported in HCT recipients with both seasonal and 2009 H1N1 influenza infection.4,7 Another shortcoming is a diagnostic bias because 95% of the seasonal influenza cohort had been tested only by culture and direct fluorescent antibody, whereas PCR testing was the main method for detecting 2009 H1N1.
In conclusion, in this direct comparison of clinical outcomes between 2009 H1N1 and seasonal influenza in high-risk immunocompromised persons, 2009 H1N1 appeared to be associated more frequently with lower respiratory tract disease and hypoxemia than seasonal influenza. There were no appreciable differences in outcome between seasonal influenza A and B. Our data suggest that early and aggressive antiviral therapy might limit LRD, mechanical ventilation, and mortality, and that even later start of antiviral may still be beneficial. The results also provide the rationale for novel and intensified approaches to influenza management. The finding that 2006 H1N1 influenza was associated with more LRD, although virtually all patients received timely preemptive antiviral therapy, suggests that more aggressive approaches should be studied, including initial combination therapy of antiviral agents with different mechanisms of action or an antiviral agent and a host cell entry blocker, such as DAS181, a sialidase fusion protein that blocks sialic acid receptors in the airway epithelium.5,40,47 Finally, our data provide the rationale for a systematic study of the role of corticosteroids in the management of influenza disease in transplant recipients and perhaps other patient populations.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Chris Davis for database services and Terry Stevens-Ayers for manuscript review.
This work was supported in part by the National Institutes of Health (grants CA18029, CA15704, and HL93294).
National Institutes of Health
Authorship
Contribution: S.-M.C. reviewed charts, analyzed data, and wrote the first draft of the manuscript; A.A.B. reviewed charts, analyzed data, and wrote the manuscript; H.X. performed the statistical analysis; J.A.E. contributed to the design and critically reviewed the manuscript; L.C. contributed to the analysis and critically reviewed the manuscript; and M.B. designed the study and analysis and wrote the final version of the manuscript.
Conflict-of-interest disclosure: J.A.E. received research funding from Sanofi Pasteur Vaccines, Novartis, MedImmune, Inc, and Adamas, Inc. M.B. received research funding from Roche Pharmaceuticals, Glaxo-Smith-Kline, and Adamas Pharmaceuticals, served as a consultant for Novartis and Roche, and on a DSMB for an influenza vaccine study funded by United States Government funds from the Office for Preparedness and Response, Biomedical Advanced Research and Development Authority, under contract to DynPort Vaccine Company. The remaining authors declare no competing financial interests.
Correspondence: Michael Boeckh, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D3-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: mboeckh@fhcrc.org.
References
Author notes
S.-M.C. and A.A.B. contributed equally to this study.