In this issue of Blood, Aguilo and coworkers demonstrate an essential role of the zinc-finger protein Prdm16 in establishment and maintenance the hematopoietic stem cell (HSC) pool.1
Hematopoiesis depends on rare stem cells, that possess the ability to self-renew and sustain life-long production of all blood lineages. Self-renewal of stem cells, and maintenance of the stem cell pool, is controlled by intricate regulatory mechanisms, balancing divergent cell fate decisions such as cell cycling, differentiation, and apoptosis. Deregulation of this balance may lead to exhaustion of the stem cell pool or to the acquisition of mutations causing malignant transformation.
One of the first genes implicated in regulation of HSC maintenance was the cell-cycle inhibitor Cdkn1a (p21). Mice lacking p21 showed essentially normal hematopoiesis but elevated cycling of HSCs leading to their premature exhaustion during hematopoietic stress.2 Similarly, mice lacking the nuclear factors Gfi1 and Pbx1 show increased cell cycling of HSCs coupled with an impaired ability to sustain the stem cell pool.3,4 Strict cell-cycle control and quiescence are therefore crucial determinants of stem cell integrity.
During recent years, accumulating evidence suggests that the quiescent state of stem cells is highly dependent on a fine-tuned regulation of their metabolic status. HSCs are thought to reside in hypoxic niches within the bone marrow, where low oxygen consumption and low production of reactive oxygen species (ROS) may promote their quiescence. Moreover, several genes known to modulate oxidative stress and ROS levels in stem cells such as the polycomb gene Bmi1 and the FoxO family of transcription factors are critically required to preserve the HSC compartment.5,6 Taken together, it is clear that diverse mechanisms contribute to the self-renewal and integrity of HSCs. However, it is not known how these mechanisms are coordinated to maintain the stem cell pool, both during steady-state hematopoiesis and in situations of hematopoietic stress.
In this context, Aguilo et al now report on the PR domain-containing 16 (Prdm16) zinc finger transcription factor (see figure).1 Intrigued by studies showing the involvement of PRDM16 in leukemic translocations and that overexpression of Prdm16 expands HSCs in vitro, Aguilo and colleagues set out to study the physiologic role of this gene in hematopoiesis.7 They started by analyzing the expression pattern of Prdm16 in various hematopoietic cell subsets and found that Prdm16 is selectively expressed in the most primitive hematopoietic stem and progenitor cells. They continued by studying the consequences of Prdm16 deficiency using gene-trap mice lacking one or both Prdm16 alleles. Homozygous knockout mice died perinatally, and is why hematopoiesis in this case was analyzed in the fetal liver. While the hematopoietic organs were essentially normal in Prdm16 deficient mice, both heterozygous and homozygous mice have a reduced number of hematopoietic stem and progenitor cells. Moreover, when transplanted in a competitive repopulation assay, the Prdm16-deficient stem cells failed to sustain robust long-term hematopoietic engraftment. Aguilo and colleagues further determined that at least part of this defect could be attributed to an increased proportion of stem and progenitor cells in active cell cycle in both heterozygous and homozygous knockout mice and in addition an increased rate of apoptosis in the stem cell compartment of homozygous mice.
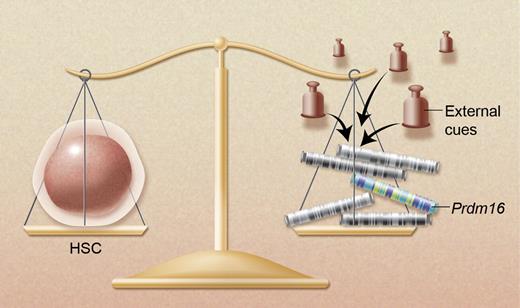
Prdm16 is one of a handful of genes that are known to be required for the maintenance and integrity of hematopoietic stem cells. Other genes include Bmi1, Evi1, Pbx1, Gfi1, and the FoxO transcription factors. The balanced activity of these nuclear factors preserves the stem cell state but is constantly challenged by external cues and stress from the environment. Professional illustration by Alice Chen.
Prdm16 is one of a handful of genes that are known to be required for the maintenance and integrity of hematopoietic stem cells. Other genes include Bmi1, Evi1, Pbx1, Gfi1, and the FoxO transcription factors. The balanced activity of these nuclear factors preserves the stem cell state but is constantly challenged by external cues and stress from the environment. Professional illustration by Alice Chen.
While Aguilo et al focused on the role of Prdm16 in stem cells of the hematopoietic system, a recent study by Morrison and colleagues used the same knockout model to demonstrate that Prdm16, in addition to its role in HSCs, is required for maintenance of neural stem cells.8 This suggests that Prdm16 may regulate stem cell function across a variety of tissues. While the work from Morrison's group implicated modulation of oxidative stress as an important mechanism behind Prdm16 function in neural stem cells, it was not clear whether a similar function existed in HSCs.
In an attempt to dissect the mechanisms of Prdm16 function in HSCs, Aguilo et al performed gene expression profiling and discovered that loss of Prdm16 results in altered levels of numerous genes known to both suppress and enhance HSC function. For example, genes that are required for normal HSC function were both up-regulated (Gata2, Pbx1) and down-regulated (Evi1, Bmi1), indicating a remarkable complexity in the interplay between these different factors. As suggested by the authors, it is certainly an intriguing possibility that Prdm16 would act as a central node in a regulatory network with positive and negative feedback loops balancing cell fate decisions of cell cycling, differentiation, and apoptosis. However, additional work will be required to clarify the mode of action by which Prdm16, directly or indirectly, influences these other HSC-related genes.
The discovery of Prdm16 as a new stem cell regulator should have an immediate and strong impact on the field of hematopoiesis and stem cell research. Few genes have been defined that regulate stem cells in an exclusive manner, not affecting other cell types. Stem cell–specific modulators such as Prdm16 should clarify the mechanisms defining the stem cell state.
While Aguilo and coworkers provide solid evidence that Prdm16 has distinct functions within the most primitive subsets of hematopoietic stem/progenitor cells, the role of Prdm16 may extend beyond normal stem cells and include cancer stem cells as well. It will be of considerable interest to manipulate Prdm16 expression in leukemia models to address whether Prdm16, or more likely its downstream targets, may influence cancer initiation and progression and thus provide new possibilities for therapeutic intervention. From another perspective, these new insights regarding stem cell self-renewal and maintenance may ultimately provide important clues for development of improved culture conditions and ex vivo expansion strategies to enhance HSC transplantation procedures.
It is clear from the study by Aguilo et al that Prdm16 belongs to an important and exclusive group of genes preserving the stem cell state and that it is a new force to be reckoned with in the field of stem cell research.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal