Abstract
The β2-integrin CD11b/CD18 mediates the firm adhesion of neutrophils (PMNs) to epithelial monolayers, a key step in PMN transepithelial migration. To complete the transmigration process, adherent PMNs must detach from epithelial monolayer surfaces to move forward. The mechanism that governs the detachment of adherent PMNs, however, is not clear. Here, we present evidence that cleavage of the CD11b extracellular domain containing the ligand-binding I-domain by 3 structural and functional related serine proteases (elastase, proteinase-3 and cathepsin G) serves as a novel mechanism for PMN detachment after the initial cell adhesion. Kinetic studies showed that the cleavage of CD11b is positively correlated with PMN detachment and subsequent transmigration. Moreover, the results demonstrated that elastase, proteinase-3 and cathepsin G all cleaved the purified, functionally active form of CD11b in a pattern similar to the CD11b shedding that occurs during PMN transmigration. Their cleavage sites on purified CD11b were located at 761Thr-Ala762 (elastase/proteinase-3) and 760Phe-Thr761 (cathepsin G), respectively. CD11b cleavage and PMN detachment and chemotaxis, were impaired in elastase/cathepsin G–deficient Beige mice; this defect could be restored by the addition of extracellular elastase. By illustrating CD11b shedding by elastase, proteinase-3 and cathepsin G as a novel mechanism for PMN detachment, our study provides novel therapeutic targets for controlling inflammation.
Introduction
A major component of many inflammatory diseases of the gastrointestinal, respiratory and urinary tracts is the migration of a large number of neutrophils (PMNs) across the epithelium and their accumulation within the lumen. Previous studies1,2 suggest that PMN migration across the epithelia and model matrices is dependent on leukocyte β2 integrin CD11b/CD18 (Mac-1, CR3), which binds to various ligands, such as ICAM-1, fibrinogen (FBG), complement protein C3bi and denatured ovalbumin.3-7 However, the mechanisms that govern CD11b/CD18-mediated PMN transmigration are still incompletely understood. One of the fundamental unsolved issues is how CD11b/CD18-bound PMNs migrate forward and detach from the substrate to which they are adhered.
Neutrophil migration is a multistep event consisting of cycles of cell adhesion (at the leading edge) and detachment (at the uropod). In general, LFA-1 and CD11b/CD18 mediate PMN adhesion. Because LFA-1 is more important than CD11b/CD18 to the efficient induction of firm leukocyte adhesion,8 CD11b/CD18 may play a critical role in adhesion strengthening and intraluminal crawling of neutrophils after initial LFA-1–mediated adhesion.9-13 By comparing the relative contributions of CD11b/CD18 and LFA-1 to the dynamics and strength of PMN adhesion to intercellular adhesion molecule 1 (ICAM-1), the investigators9-11 found that PMN adhesion appears to be a cooperative and sequential process of LFA-1–dependent capture followed by CD11b/CD18-mediated stabilization. CD11b/CD18–dependent crawling of adherent PMNs after initial LFA-1–mediated adhesion has been identified as a new critical step in the PMN recruitment cascade.10,11 For adherent PMNs to crawl, the PMN surface molecules (CD11b/CD18) must disengage from their binding ligands on the epithelia or tissue matrix. However, the mechanism(s) that govern the detachment of adherent PMNs are largely unknown. Because neutrophil elastase (NE), proteinase-3 (NPR-3) and cathepsin G (NCG) can specifically bind to CD11b/CD18,14 these leukocyte serprocidins (serine proteases with microbicidal activities) may play roles in PMN de-adhesion by competing for CD11b/CD18 binding with epithelial ligands, or by enzymatically cleaving the extracellular domain of CD11b/CD18. To date, the shedding of LFA-1,15 L-selectin,16 and integrin-binding proteins, such as ICAM-1,17 during leukocyte rolling and transmigration have been reported.
Neutrophil serprocidins, including NE, NCG, and NPR-3, have been widely shown to play important roles in modulating inflammation, particularly leukocyte chemotaxis.18-21 Although it is generally believed that leukocyte serprocidins promote PMN transmigration by cleaving off the adhesion molecules that hold PMN on the cell migration path, the molecular basis of this role for serprocidins is still unclear, and the reports about the functions of serprocidins in leukocyte recruitment remain controversial. Using NE-deficient mice and various inflammatory mouse models, systematic studies from Nourshargh's laboratory demonstrated the essential role of NE in mediating PMN transmigration.19,20,22 In contrast, Hirche et al23 reported no significant defects of leukocyte transmigration in mice with individual NE knockouts. Similarly, the inhibition of NE by a specific inhibitor did not affect leukocyte transendothelial migration under flow.24 Tkalcevic et al25 also reported normal PMN development and recruitment in mice deficient in NE or NCG, despite impaired immunity and enhanced resistance to endotoxin because of the lack of an individual serine protease. A study by Belaaouaj et al26 using NE-deficient mice also indicated that the absence of NE impaired the intracellular killing of bacteria without affecting PMN migration.
In this study, we present the first evidence that the shedding of the PMN surface CD11b protein by 3 neutrophil serprocidins (NE, NCG, and NPR-3) plays a critical role in PMN detachment during transmigration. Blocking CD11b cleavage with a combination of inhibitors directed against the 3 serprocidins completely inhibited PMN detachment and its subsequent migration. These findings define a new model for the regulation of PMN adhesion and detachment, and provide novel targets for therapeutic strategies aimed at attenuating pathologic mucosal inflammation.
Methods
Cells
Normal human PMNs were isolated from the peripheral blood of healthy volunteers using Ficoll/dextran sedimentation.27 Isolated PMNs were resuspended at a concentration of 5 × 107 cells/mL in Hanks Balanced Salt Solution lacking Ca2+ and Mg2+(HBSS−). PMN were also obtained from the purulent exudates (PUS) of 3 patients after therapeutic drainage of an axillary sweat gland abscess at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China). The Institutional Review Board of Georgia State University and Nanjing University approved all protocols involving human blood or body fluid management, and written informed consent was obtained from each participant in accordance with the Declaration of Helsinki. Mouse bone marrow (BM) PMNs were obtained by flushing the femoral and tibial cavities with PBS.
CD11b/CD18-serprocidin binding
Binding was assessed according to the method described previously28,29 with minor modifications. For assaying CD11b/CD18–serprocidin binding, human leukocyte serprocidins and azurocidin were immobilized onto 96-well microtiter plates. The plates were blocked with 1% BSA in HBSS. CD11b/CD18 at various concentrations in HBSS containing 0.1% Triton X-100 was added to the wells and incubated for 1 hour at 37°C in the absence or presence of various inhibitors/antibodies. BSA-coated wells served as blank controls. The bound CD11b was detected by incubation with mAb LM2/1 (10 μg/mL) followed by HRP-secondary antibody incubation and colorimetric measurement after adding substrate (ABTS). For assaying the link between the functional status of CD11b/CD18 and CD11b/CD18–serprocidin binding, purified CD11b/CD18 was immobilized onto microtiter plates. After blocking with 1% BSA, 10 μg/mL NE or CBRM1/5 mAb was added to the wells for 1 hour at 37°C in the presence or absence of 2.5mM EDTA. The bound NE or CBRM1/5 was detected by incubation with 10 μg/mL of HRP-conjugated anti-NE antibody or anti–mouse IgG followed by addition of ATBS and colorimetric measurement.
Zigmond chamber assay for PMN migration and detachment
Isolated PMNs were loaded in the center of a cover glass coated with fibrinogen (FBG), and placed upside-down onto a Zigmond chamber (Neuroprobe).30,31 The chamber was set on a heating stage (37°C) with 5% CO2. After 5 minutes, an fMLP gradient (10−7M for human PMNs and 5 × 106M for mouse PMNs) was added to induce PMN adhesion. Time-lapse video images were used to track the positions of the PMNs. The final position of a PMN relative to its starting position was calculated, and these data were graphed. On these graphs, a positive distance reflects travel up the fMLP gradient. The detachment kinetics of the individual adherent PMNs was also characterized using the Zigmond chamber. The kinetics of the detachment of individual adherent PMNs was further characterized using the Zigmond chamber.30,31 In the experiment, PMNs were initially allowed to adhere to FBG-coated cover glass on the Zigmond chamber by applying an fMLP gradient. The movement of 12 randomly selected PMNs for each condition was tracked and the number of cells that show the “first” detachment was recorded at 0-to-50–second time frames. The detachment of PMN was determined by the contraction of the “tail” of the PMN along the migration path. The cell tracking experiment for each condition was repeated 8 times.
The kinetics of CD11b shedding and PMN detachment
FBG (Sigma-Aldrich) was immobilized onto 48-well Terasaki plates (Robbins Scientific) and nonspecific protein binding was blocked with HBSS containing 1% BSA. PMNs (5 × 105) from human peripheral blood or mouse bone marrow were added to the wells containing 100 μL HBSS, and after 5 minutes, fMLP (10−7M for human PMNs and 5 × 10−6M for mouse PMNs) was added to promote PMN adhesion. At different incubation times, plates were washed with HBSS 3 times. Bound PMNs were then quantified by MPO assay28,29 and examined for CD11b shedding by Western blot using the R7928A antibody. Detached PMNs were also collected and analyzed for CD11b shedding. Antibodies were added before the cell attachment induced by fMLP. The kinetics of detachment of individual adherent PMN was further characterized using Zigmond chamber.30,31
Mouse zymosan peritonitis
Adult C57BL/6J mice (aged 8-10 weeks) were purchased from the Model Animal Research Center. Beige mice (C57BL/6J-Lystbg−J/J) were obtained from The Jackson Laboratory. A zymosan-induced mouse intraperitoneal inflammation model was established as previously described.32 Briefly, beige mice and control C57BL/6J mice were injected intraperitoneally with zymosan A (Sigma-Aldrich; 1 mg in 0.5 mL sterile saline). The animals were killed 3 hours later and the peritoneal cavities were lavaged with 5 mL of PBS containing 2.5mM EDTA. Normally, nearly 90% of the cells recovered by this procedure are granulocytes. For AEBSF treatment, mice were administered AEBSF (180 μg/kg) via peritoneal injection 2 hours before zymosan injection.
Results
Shedding of CD11b/CD18 during PMN detachment and migration
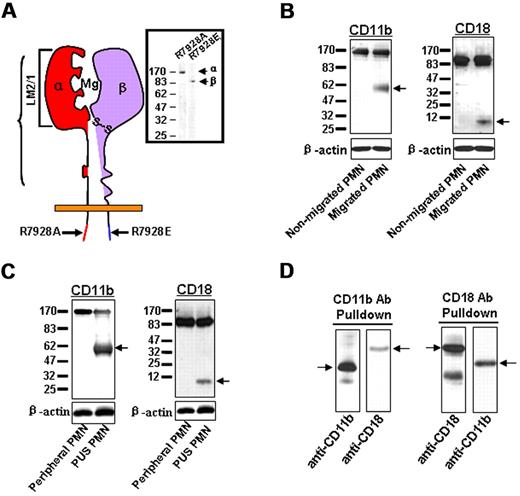
CD11b/CD18 is a major β2 integrin in PMNs, and its heterodimeric structure consists of CD11b (α) and (β) subunits. During PMN transepithelial migration, CD11b/CD18 mediates PMN adhesion to epithelial basolateral surfaces through the interactions between the CD11b inserted domain (I-domain) and its epithelial counter-receptor(s). To study CD11b/CD18–mediated leukocyte chemotaxis, several monoclonal and polyclonal antibodies against various domains of human CD11b and CD18 subunits were used to detect the structural and conformational changes of the β2 integrin. As shown in Figure 1A, antibodies LM2/1, R7928A and R7928E recognized the I-domain–containing extracellular domain of CD11b, the C-terminal tail of CD11b and the CD18 subunit, respectively. Western blot analysis showed that antibodies R7928A and R7928E recognized protein bands at 170 kD (CD11b) and 95 kD (CD18), respectively, from PMNs that were freshly isolated from the peripheral blood of healthy donors. However, when used to blot the cell lysates of PMNs that migrated across inverted epithelial T84 monolayers in response to an f-Met-Leu-Phe (fMLP) gradient, we consistently detected an additional smaller band at approximately 60 kD (Figure 1B). Because the R7928A antibody reacted with the cytoplasmic tail of CD11b regardless of any modifications in the extracellular domain, one possible explanation for these results is that a large portion of the extracellular domain (∼ 110 kD) of CD11b was cleaved off during PMN transepithelial migration. Recently, Vaisar et al33 reported that nearly the entire extracellular domain of the LFA-1 CD18 subunit was cleaved by metalloproteinase-9 (MMP-9) from macrophages. Our data also show that most of the extracellular domain of the CD18 subunit from CD11b/CD18 was cleaved during PMN transmigration. In addition to the intact 95 kDa band, the R7928E antibody detected a band at ∼ 6 kDa in the lysate of migrated PMNs, but not in the lysate of nonmigrated PMNs. We performed the migration assay of PMNs across collagen-coated Transwell filters, and observed both the 60 kD and 170 kD CD11b bands in the lysate of migrated PMNs; however, only the 170 kD band was detected in the lysate of nonmigrated PMNs (data not shown).
Shedding of the CD11b subunit during PMN transmigration. (A) Binding regions of antibodies against CD11b or CD18. (B) Cleavage of CD11b and CD18 in PMNs migrating across inverted T84 monolayers. (C) Cleavage of CD11b and CD18 in PMNs isolated from human PUS. (D) Partial association of the soluble cleaved CD11b and CD18 fragments in the supernatant of the migrating PMNs. Data represent 3 or more independent experiments.
Shedding of the CD11b subunit during PMN transmigration. (A) Binding regions of antibodies against CD11b or CD18. (B) Cleavage of CD11b and CD18 in PMNs migrating across inverted T84 monolayers. (C) Cleavage of CD11b and CD18 in PMNs isolated from human PUS. (D) Partial association of the soluble cleaved CD11b and CD18 fragments in the supernatant of the migrating PMNs. Data represent 3 or more independent experiments.
Next, we tested whether the cleavage of CD11b/CD18 occurs in vivo during PMN recruitment under inflammatory conditions. Samples of purulent exudates (PUS) from patients were obtained after therapeutic drainage of the axillary sweat gland abscess. Microscopic examination of the purulent material revealed that it consisted of > 80% PMNs. As shown in Figure 1C, lysates made from PMNs obtained from the PUS revealed a strong 60 kD band and a relatively weak 170-kD band, indicating that significant amounts of the PMN CD11b extracellular domain were cleaved in the abscess sample. Similarly, significant CD18 cleavage was also detected in PMNs obtained from the PUS, but not in PMNs from peripheral blood.
Cross-immunoprecipitation using anti-CD11b and anti-CD18 antibodies (Figure 1D) further suggested that the cleaved CD11b and CD18 fragments in the supernatant were at least partially associated with one another, suggesting a potential biologic function for the soluble integrin fragments.
We surmised that if the CD11b extracellular domain were cleaved during PMN migration, then the number of cleaved CD11b and CD18 fragments would positively correlate with the PMN transmigration capacity. Thus, we analyzed both the cleaved CD11b and CD18 fragments in the supernatant and extracellular matrix. PMN migration across epithelial T84 monolayers was terminated at various time points. T84 monolayers were washed twice, and the washing solution was combined with the supernatants from the migration setup. After ultracentrifugation (110 000g, 2 hours) to remove cell debris and cell-derived microvesicles, the solution was concentrated using a 30 000-MW cutoff filter (Centricon). As shown in Figure 2, the increase in cleaved CD11b and CD18 fragments detected by ELISA (Figure 2A) positively correlated with the extent of PMN transmigration (Figure 2B). Western blot analysis using an antibody against the CD11b extracellular domain (hMAC-ex) directly revealed the presence of the cleaved CD11b fragment in the supernatant of the PMN migration (Figure 2C). We also blotted the supernatant with R7928A (an antibody recognizing the C-terminus of CD11b), but obtained no signal (data not shown); this result indicates that the CD11b fragments in the supernatant contain no CD11b intracellular C-terminus, and are indeed cleaved from the PMN surfaces. Interestingly, NE and NCG, 2 major serprocidins normally stored in PMN azurophil granules, were detected in the supernatant of transmigrating PMNs (Figure 2C). This observation is consistent with previous reports that elastase is a part of the extracellular network released from migrating PMNs.34,35
Correlation between CD11b/CD18 shedding and PMN transmigration. (A) Soluble cleaved CD11b and CD18 fragments detected in PMN migration medium by ELISA (bottom panel) using mAbs LM2/1 and MEM-148, respectively. The isotype matched mouse IgG1 was used as the control. (B) PMN transepithelial migration during the same time frame. (C) Western blot analysis of the CD11b fragment and leukocyte serine proteases (NE and NCG) in the concentrated supernatant of the PMN migration set. Data are presented as mean ± SD (n = 4). *P < .05; **P < .01.
Correlation between CD11b/CD18 shedding and PMN transmigration. (A) Soluble cleaved CD11b and CD18 fragments detected in PMN migration medium by ELISA (bottom panel) using mAbs LM2/1 and MEM-148, respectively. The isotype matched mouse IgG1 was used as the control. (B) PMN transepithelial migration during the same time frame. (C) Western blot analysis of the CD11b fragment and leukocyte serine proteases (NE and NCG) in the concentrated supernatant of the PMN migration set. Data are presented as mean ± SD (n = 4). *P < .05; **P < .01.
The binding of CD11b/CD18 leads to the generation of both inside-out and outside-in signals that change PMN functional status.36,37 After binding to its ligand(s), CD11b/CD18 may undergo a conformational change. Therefore, we determined whether the cleavage of PMN surface CD11b depends on CD11b/CD18–mediated adhesion. For this experiment, we compared the CD11b patterns in PMNs after different treatments. As shown in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article), a single 170 kDa band (intact CD11b) was observed in freshly isolated PMNs (lane 1) and PMNs suspended in HBSS– solution containing 10−7M PMA (lane 2), while a 60 kDa cleaved CD11b fragment was clearly observed in PMNs after fMLP-induced adhesion to immobilized FBG (lane 3). Blocking fMLP-induced PMN adhesion with the inhibitory anti-CD11b antibody 44a (lane 4) and EDTA (lane 5) abolished the shedding of PMN CD11b. These results suggest that the cleavage of the CD11b extracellular domain depends on CD11b/CD18–mediated PMN adhesion.
Role of serprocidins in CD11b shedding, PMN detachment and subsequent migration
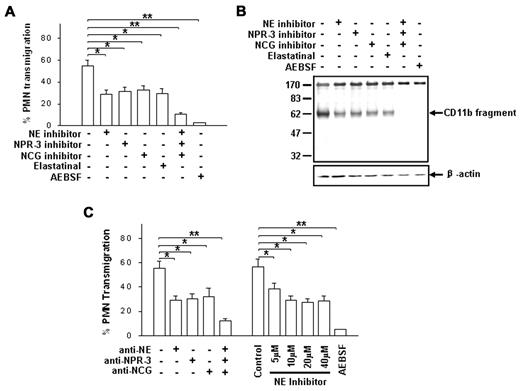
To show that CD11b proteolytic cleavage is essential for PMN detachment and subsequent migration, we further tested whether blocking CD11b cleavage prevents PMN transmigration. Because PMNs normally store various proteases in distinct granules and can release them onto a surface in response to a chemoattractant,38 we screened a panel of specific inhibitors and inhibitory antibodies against leukocyte proteases for blocking both PMN chemotaxis and CD11b shedding. As shown in Figure 3, specific inhibitors against NE, NPR-3 and NCG significantly reduced both PMN transepithelial migration (Figure 3A) and CD11b shedding (Figure 3B). In the absence of the inhibitors, more than half of the total PMNs rapidly migrated across epithelial T84 monolayers after 1 hour, and a significant amount of 60 kD CD11b remnant was detected in the migrating PMNs. In the presence of individual inhibitors of NE, NPR-3 and NCG (10μM each), PMN transmigration was inhibited by 49.4% ± 6.1%, 41.6% ± 5.7% and 45.8% ± 7.4%, respectively. The partial inhibition of CD11b shedding by an individual protease inhibitor was confirmed by Western blot analysis. However, when the 3 specific inhibitors of NE, NPR-3 and NCG were combined, both PMN transmigration and CD11b shedding were completely blocked. The total inhibition of PMN transmigration and CD11b shedding were obtained by the addition of 50μM AEBSF, a potent water-soluble serine protease inhibitor. Similar inhibitions of PMN transmigration were obtained in the presence of specific antibodies against each protease (Figure 3C). Increasing the inhibitor concentration further tested partial inhibition of PMN transmigration by individual protease inhibitors. We found that PMN transmigration was not completely blocked by the NE inhibitor, even at high concentrations (Figure 3C). Together, these results demonstrate a correlation between PMN transmigration and CD11b shedding, and indicate critical roles for the 3 leukocyte serprocidins in CD11b proteolytic cleavage and PMN transmigration.
Identification of 3 PMN serprocidins as protease candidates in cleaving CD11b and promoting PMN transmigration. (A-B) Effects of various serine protease inhibitors (10μM each) on fMLP-induced PMN chemotaxis and CD11b cleavage, respectively. (C) Effects of anti-serprocidin antibodies (50 μg/mL each) and NE inhibitors on PMN transmigration. AEBSF (50μM) was used as positive control. Data are presented as mean ± SD (n = 4). *P < .05; **P < .01.
Identification of 3 PMN serprocidins as protease candidates in cleaving CD11b and promoting PMN transmigration. (A-B) Effects of various serine protease inhibitors (10μM each) on fMLP-induced PMN chemotaxis and CD11b cleavage, respectively. (C) Effects of anti-serprocidin antibodies (50 μg/mL each) and NE inhibitors on PMN transmigration. AEBSF (50μM) was used as positive control. Data are presented as mean ± SD (n = 4). *P < .05; **P < .01.
Next, we tested whether leukocyte serprocidin-mediated PMN CD11b shedding correlates with the detachment of adherent PMNs. In these experiments, the PMNs were induced to bind to FBG-coated plates in the presence or absence of leukocyte serprocidin inhibitors and inhibitory antibodies. At different time points, the plates were gently washed with HBSS, and the cells were assessed by myeloperoxidase (MPO) assay. As shown in Figure 4A, fMLP stimulation led to enhanced PMN adhesion via CD11b/CD18-FBG binding in the initial 5 minutes, which was followed by gradual PMN detachment. Interestingly, the addition of 25 μg/mL anti-NE antibody (NP57) resulted in a significant delay in PMN detachment from the immobilized FBG after the initial adhesion. The detachment of the adherent PMNs was completely inhibited by AEBSF. Western blot analysis showed that CD11b shedding occurred in the absence of NP57 or AEBSF, suggesting that CD11b shedding may serve as a mechanism of PMN disengagement from the substratum (Figure 4B). As expected, CD11b shedding during PMN detachment was significantly blocked by NP57 and AEBSF. These results demonstrated a direct link between CD11b shedding by leukocyte serprocidins and the detachment of adherent PMNs. To further analyze the kinetics of individual cell detachment, we performed an adhesion and detachment assay on a Zigmond chamber.30,31 In this experiment, we tracked the movement of randomly selected cells (∼12 cells per condition for a single assay) after fMLP-induced adhesion. The initial detachment of individual cells was detected by observing the contraction of the ‘tail’ along the migration path. Because PMNs normally follow a dynamic cycle of adhesion, detachment, and re-adhesion in response to an fMLP gradient, we recorded the number of cells that ‘initially’ detached after the application of fMLP within a 50-second time frame. As shown in Figure 4C, nearly all of the 12 randomly selected adherent PMNs had their initial detachment within 40 seconds in the absence of inhibitors, whereas PMN detachment was significantly slowed by the addition of NP57 and AEBSF. Morphologically, PMNs stimulated by fMLP in the presence of the AEBSF generally had an overextended “tail” (Figure 4C inset arrowhead) compared with fMLP-stimulated PMNs in the absence of AEBSF.
Effects of leukocyte serprocidins on the cleavage of CD11b and the detachment of adherent PMNs from immobilized FBG. (A) The detachment of adherent PMNs. The fMLP (10−7M)–induced PMN adhesion to immobilized FBG was performed in the presence of NE inhibitor (10μM), anti-NE mAb NP57 (50 μg/mL) or serine protease inhibitor ABESF (50μM). (B) The cleavage of CD11b by NE or other serprocidins. All PMNs (adherent or detached) were collected and blotted with antibody R7928A. Data represent 3 or more independent experiments. (C) “Initial” detachment of individual adherent PMNs measured using the Zigmond chamber assay (n = 8). (Inset) Phase contrast images of migrating PMNs in the presence (top) or absence (bottom) of AEBSF. Note that in the presence of AEBSF, PMN has a typical extended “tail” (arrowhead).
Effects of leukocyte serprocidins on the cleavage of CD11b and the detachment of adherent PMNs from immobilized FBG. (A) The detachment of adherent PMNs. The fMLP (10−7M)–induced PMN adhesion to immobilized FBG was performed in the presence of NE inhibitor (10μM), anti-NE mAb NP57 (50 μg/mL) or serine protease inhibitor ABESF (50μM). (B) The cleavage of CD11b by NE or other serprocidins. All PMNs (adherent or detached) were collected and blotted with antibody R7928A. Data represent 3 or more independent experiments. (C) “Initial” detachment of individual adherent PMNs measured using the Zigmond chamber assay (n = 8). (Inset) Phase contrast images of migrating PMNs in the presence (top) or absence (bottom) of AEBSF. Note that in the presence of AEBSF, PMN has a typical extended “tail” (arrowhead).
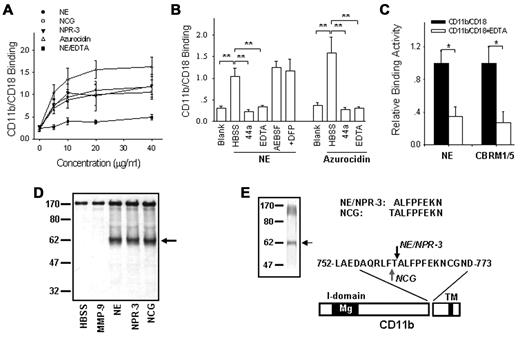
We next characterized the interactions between leukocyte serprocidins and CD11b/CD18. As shown in Figure 5A, NE, NPR-3 and NCG all specifically bound to CD11b/CD18, which is consistent with the previous finding by Cai et al14 Interestingly, azurocidin, which shares a high sequence homology with NE but is proteolytically inactive,39 bound to CD11b/CD18 more than the 3 serprocidins. This result suggests that the enzymatic activity of NE is not necessary for CD11b/CD18 binding. Indeed, the protease inhibitors AEBSF and DFP did not affected the binding of the 3 serprocidins to CD11b/CD18 (Figure 5B), confirming that enzymatic protease activity does not contribute to CD11b/CD18 binding. In fact, the relatively low CD11b/CD18-binding capacity of the 3 serprocidins compared with that of azurocidin might be because of the cleavage of CD11b by the serprocidins after protein binding. The binding of NE to CD11b/CD18 was abolished by anti-CD11b antibody 44a and by deactivating CD11b/CD18 with EDTA. Interestingly, using CBRM1/5, a mAb specific for the activation epitope on CD11b,40-42 revealed that the binding of NE to CD11b/CD18 reflects expression of a functionally active CD11b/CD18 (Figure 5C).
Cleavage of purified, functionally active CD11b/CD18 by PMN serprocidins. (A) Binding of PMN serprocidins with purified CD11b/CD18. (B) Binding of PMN serprocidins and azurocidin with purified CD11b/CD18 (20 μg/mL) in the presence or absence of various inhibitors. (C) Correlation between NE-CD11b/CD18 binding and CRBM1/5-CD11b/CD18 binding. In this experiment, CD11b/CD18 was first immobilized onto the plates, incubated with NE and fluorescently labeled CBRM1/5. (D) Specific cleavage of purified, functionally active CD11b/CD18 by the 3 leukocyte serprocidins, but not by MMP-9. (E) Identification of the cleavage site of NE, NPR-3 and NCG on CD11b. (Left panel) Gelcode staining of the cleavage of purified CD11b by serprocidins. (Top panel) The N-terminal peptide sequence of the ∼ 60 kD CD11b fragment cleaved by NE, NPR-3, and NCG, respectively. (Bottom panel) The location of the cleavage sites within the extracellular domain of CD11b. Note that NE and NPR-3 cleave CD11b at the same site between 761Thr-Ala763 (red arrow), while NCG cleaves CD11b between 760Phe-Thr761 (blue arrow). Data represent the mean ± SD of 3 independent experiments, each performed in triplicate. *P < .05; **P < .01.
Cleavage of purified, functionally active CD11b/CD18 by PMN serprocidins. (A) Binding of PMN serprocidins with purified CD11b/CD18. (B) Binding of PMN serprocidins and azurocidin with purified CD11b/CD18 (20 μg/mL) in the presence or absence of various inhibitors. (C) Correlation between NE-CD11b/CD18 binding and CRBM1/5-CD11b/CD18 binding. In this experiment, CD11b/CD18 was first immobilized onto the plates, incubated with NE and fluorescently labeled CBRM1/5. (D) Specific cleavage of purified, functionally active CD11b/CD18 by the 3 leukocyte serprocidins, but not by MMP-9. (E) Identification of the cleavage site of NE, NPR-3 and NCG on CD11b. (Left panel) Gelcode staining of the cleavage of purified CD11b by serprocidins. (Top panel) The N-terminal peptide sequence of the ∼ 60 kD CD11b fragment cleaved by NE, NPR-3, and NCG, respectively. (Bottom panel) The location of the cleavage sites within the extracellular domain of CD11b. Note that NE and NPR-3 cleave CD11b at the same site between 761Thr-Ala763 (red arrow), while NCG cleaves CD11b between 760Phe-Thr761 (blue arrow). Data represent the mean ± SD of 3 independent experiments, each performed in triplicate. *P < .05; **P < .01.
Based on the specific interaction between the serprocidins and CD11b/CD18, we further tested whether the purified, functionally active CD11b/CD18 could be cleaved by PMN serprocidins in a similar manner during PMN detachment or transmigration. In this experiment, CD11b/CD18 purified from human PMNs was incubated with human NE, NPR-3, NCG, and matrix metallopeptidase 9 (MMP-9) under the appropriate conditions for 30 minutes. As shown in Figure 5D, the CD11b subunit of CD11b/CD18 was cleaved by NE, NPR-3 and NCG, but not by MMP-9. More surprisingly, all 3 PMN serprocidins had the same digestion pattern, which was virtually indistinguishable from that observed in migrating PMNs. The N-terminal sequence of the 60 kDa CD11b fragment after cleavage by the 3 serprocidins (Figure 5E left arrow), further confirmed that the cleavage sites on CD11b for NE, NCG and NPR-3 were close. The N-terminal sequence of the 60 kD CD11b fragment after cleavage by NE or NPR-3 was ALFPFEKN, indicating that NE and NPR-3 had the same CD11b cleavage site located between 761Thr-Ala762. For NCG, the N-terminal sequence was TALFPFEKN, indicating that the cleavage site was located between 760Phe-Thr761. The 2 CD11b cleavage sites were separated by a single amino acid. The cleavage of purified CD11b/CD18 by the PMN serprocidins further supports the notion that the shedding of CD11b is a specific and necessary event during PMN detachment and subsequent migration and depends on CD11b/CD18-serprocidin binding interactions.
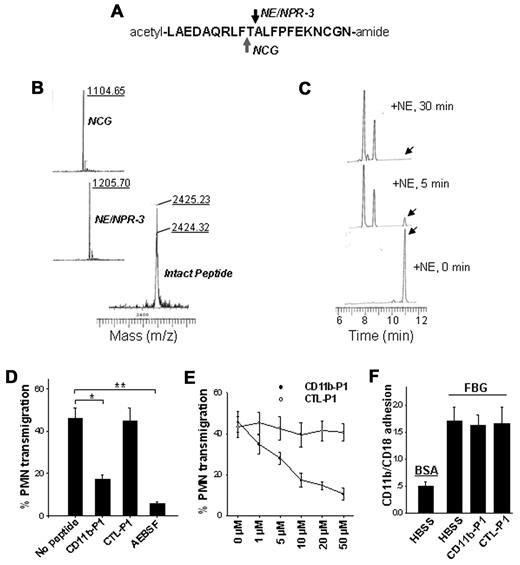
The cleavage of CD11b was further confirmed using CD11b-P1, a synthetic peptide matching the shed region of CD11b. As shown in Figure 6A, CD11b-P1 was incubated with commercially available neutrophil serprocidins. Mass spectrometry confirmed the cleavage of a 20-mer peptide by NE, NPR-3 and NCG (Figure 6B). The 3 PMN serprocidins cleaved the peptide approximately in the middle, revealing the specificity of the CD11b cleavage. We also studied the kinetics of peptide cleavage by NE using reverse-phase HPLC analysis. NE rapidly cleaved the peptide into 2 fragments with similar sizes (Figure 6C). After 5 minutes of incubation, the majority of the peptide was already cleaved (the intact peptide peak is indicated by an arrow).
Cleavage of the synthetic peptide overlapping the CD11b cleavage region by the 3 serprocidins and the specific inhibition of PMN transmigration by the CD11b-derived peptide. (A) A 20-amino-acid peptide (20-mer) with a random sequence (CTL-P1) or a sequence matching the cleavage region of CD11b (CD11b-P1) was synthesized. (B) Mass spectrometry analysis of CD11b-P1 cleavage by NE, NPR-3, and NCG, respectively. The synthetic peptides were incubated with each serprocidin for 30 minutes under appropriate conditions. (C) Reverse-phase HPLC analysis of the kinetics of CD11b-P1 cleaved by NE. Note that the peak of the intact peptide (indicated by the arrow) rapidly disappeared and shifted to that of the smaller peptide with a running time of 8.0-9.0. (D-E) The effects of synthetic peptides on fMLP-induced migration of human PMNs across collagen-coated Transwell filters. (F) No effect of synthetic peptides on FBG-CD11b/CD18 binding. Data represent the mean ± SD of 3 independent experiments, each performed in triplicate. *P < .05; **P < .01.
Cleavage of the synthetic peptide overlapping the CD11b cleavage region by the 3 serprocidins and the specific inhibition of PMN transmigration by the CD11b-derived peptide. (A) A 20-amino-acid peptide (20-mer) with a random sequence (CTL-P1) or a sequence matching the cleavage region of CD11b (CD11b-P1) was synthesized. (B) Mass spectrometry analysis of CD11b-P1 cleavage by NE, NPR-3, and NCG, respectively. The synthetic peptides were incubated with each serprocidin for 30 minutes under appropriate conditions. (C) Reverse-phase HPLC analysis of the kinetics of CD11b-P1 cleaved by NE. Note that the peak of the intact peptide (indicated by the arrow) rapidly disappeared and shifted to that of the smaller peptide with a running time of 8.0-9.0. (D-E) The effects of synthetic peptides on fMLP-induced migration of human PMNs across collagen-coated Transwell filters. (F) No effect of synthetic peptides on FBG-CD11b/CD18 binding. Data represent the mean ± SD of 3 independent experiments, each performed in triplicate. *P < .05; **P < .01.
Cleavage of the synthetic peptide by leukocyte serprocidins confirmed the location of the cleavage site on CD11b and demonstrated that small specific peptides are potential reagents that can compete for CD11b binding and subsequent CD11b cleavage. Next, we tested whether the synthetic peptides could reduce PMN transmigration by inhibiting the PMN serprocidins-cleavage of CD11b. As shown in Figure 6D, 10μM CD11b-P1 significantly blocked PMN transmigration, while the control peptide with a random sequence (CTL-P1) had no effect. The inhibition of PMN transmigration by CD11b-P1 was also dose-dependent (Figure 6E). Interestingly, although both both serprocidins and CD11b ligand FBG bind to CD11b I-domain, the cleavage sites of serprocidins are located at the membrane proximal region of CD11b, and CD11b-P1 had no effect on FBG-CD11b/CD18 binding (Figure 6F).
Using immunofluorescence and FACS, we determined the expression and localization of the PMN serprocidins and CD11b/CD18 with or without an fMLP stimulus. As shown in supplemental Figure 2, PMNs had a low level of surface expression of both CD11b/CD18 and NE under resting conditions. After 30 minutes of stimulation with fMLP, the PMN cell surface expression of both CD11b and NE was significantly increased. Strikingly, NE was generally colocalized with CD11b on one side of the migrating PMNs, probably the uropod (supplemental Figure 2A arrowhead). Surface double labeling showed that CD11b was not only colocalized with NE possibly at the uropod of PMNs, but also that CD11b was concentrated at another side of the migrating PMNs, probably at the leading edge (supplemental Figure 2A arrows) where no NE labeling was observed. Similar results were obtained using antibodies against NPR-3 and NCG (images not shown). We also determined the time-course of PMN surface expression of the 3 serprocidins and CD11b/CD18 after fMLP stimulation (supplemental Figure 2B). The up-regulation of the surface expression of CD11b by fMLP was significantly faster than that of the PMN serprocidins.
The role of CD11b shedding in murine PMN detachment and transmigration
Next, CD11b cleavage and its role in modulating PMN transmigration were examined using a mouse inflammation model. First, we tested whether similar CD11b shedding occurs during the chemotaxis of murine PMNs. In this experiment, mouse PMNs were collected by peritoneal lavage at 3 hours after zymosan injection.32 As shown in Figure 7A, Western blot analysis using a polyclonal anti–mouse CD11b C-terminus antibody (MCT33) clearly showed the cleavage of CD11b in migrating PMNs but not nonmigrated PMNs from bone marrow (BM). The shedding pattern of mouse CD11b was similar to that observed in migrating human PMNs.
Effects of serprocidins on mouse PMN CD11b shedding and PMN detachment during chemotaxis. (A) Cleavage of CD11b in migrated PMNs collected from peritoneal lavages of mice with zymosan peritonitis. PMNs from mouse BM served as controls. (B) Effects of serine protease inhibitors on mouse PMN influx during zymosan peritonitis. PMNs were collected at 3 hours after zymosan injection. (C) Effects of serine proteases and protease inhibitors on mouse PMN transfilter migration. The transmigration of BM PMNs was induced by 5 × 10−6M fMLP. (Inset) Cleavage of PMN CD11b during PMN transfilter migration. Note that beige mouse PMNs had a significantly less CD11b cleavage. (D) Relative migration speed of mouse PMNs detected using the Zigmond chamber assay. PMN migration along immobilized FBG in the presence or absence of protease inhibitors (10μM each) or antibody (25 μg/mL) was measured within 30 minutes. (E) The impairment of PMN detachment in beige mice and the restoration of beige mouse PMN detachment by adding 1μM extracellular elastase (n = 8). Data represent the mean ± SD of 3 independent experiments, each performed in triplicate. *P < .05; **P < .01.
Effects of serprocidins on mouse PMN CD11b shedding and PMN detachment during chemotaxis. (A) Cleavage of CD11b in migrated PMNs collected from peritoneal lavages of mice with zymosan peritonitis. PMNs from mouse BM served as controls. (B) Effects of serine protease inhibitors on mouse PMN influx during zymosan peritonitis. PMNs were collected at 3 hours after zymosan injection. (C) Effects of serine proteases and protease inhibitors on mouse PMN transfilter migration. The transmigration of BM PMNs was induced by 5 × 10−6M fMLP. (Inset) Cleavage of PMN CD11b during PMN transfilter migration. Note that beige mouse PMNs had a significantly less CD11b cleavage. (D) Relative migration speed of mouse PMNs detected using the Zigmond chamber assay. PMN migration along immobilized FBG in the presence or absence of protease inhibitors (10μM each) or antibody (25 μg/mL) was measured within 30 minutes. (E) The impairment of PMN detachment in beige mice and the restoration of beige mouse PMN detachment by adding 1μM extracellular elastase (n = 8). Data represent the mean ± SD of 3 independent experiments, each performed in triplicate. *P < .05; **P < .01.
To test whether mouse leukocyte serprocidins also play critical roles in mouse CD11b shedding and PMN transmigration, we examined the effect of serine protease inhibitors on mouse PMN influx. Because beige mice are elastase- and cathepsin G–deficient,43 they were also used to test CD11b shedding and PMN transmigration under inflammatory conditions. As shown in Figure 7B, zymosan-induced PMN influx in C57BL/6J wild-type mice was completely blocked by AEBSF, suggesting the critical role of serine proteases in the intraperitoneal accumulation of mouse PMNs during zymosan-induced acute peritonitis. As expected, elastase- and cathepsin G–deficient beige mice exhibited an impaired PMN chemotaxis after zymosan treatment. The migration of mouse PMNs across collagen-coated Transwell filters was also reduced by AEBSF and a combination of inhibitors against mouse NE, NCG and NPR-3 (Figure 7C). PMNs from the beige mice also exhibited an impaired fMLP-induced transfilter migration, consistent with the reduced CD11b shedding in PMNs from beige mice (Figure 7C inset). However, this impaired transmigration can be largely reversed by the addition of mouse elastase (mNE). The role of leukocyte serprocidins in regulating CD11b/CD18–mediated PMN chemotactic transmigration was further analyzed using Zigmond chambers30,31 (Figure 7D). In the presence of an fMLP gradient, inhibitors against individual serprocidins, AEBSF, EDTA and antibody 44a decreased the migration speed of mouse PMNs along an FBG-coated surface. PMNs from beige mice also had lower migration speeds than PMNs from wild-type mice, and this impairment of migration could be largely reversed by the addition of extracellular mNE. The detachment of individual adherent PMNs in the Zigmond chamber assay further showed that PMNs from NE- and NCG-deficient beige mice exhibited an impaired detachment after CD11b/CD18-FBG binding; this impairment of detachment could also be reversed by the addition of mNE (Figure 7E). As expected, the detachment of adherent beige mouse PMNs was blocked by AEBSF.
Discussion
CD11b shedding is critical for PMN detachment and transmigration
The detachment of adherent PMNs is a critical step in PMN transepithelial migration. After CD11b/CD18-mediated adhesion to the epithelial monolayers, PMNs must detach from the epithelial cells to migrate forward. Such locomotive phenomena exhibited by neutrophils10 and monocytes44 along the endothelial monolayer surface were previously well defined. In general, many adhesion and de-adhesion steps are required for the leukocyte to reach the inflammation site.45,46 Specifically, the detachment of CD11b/CD18 from its binding ligand(s) is necessary for PMN intralumenal crawling. However, the mechanism that governs the release of PMNs from CD11b/CD18–mediated cell adhesion remains unknown.
Several lines of evidence derived from the present study indicate that CD11b shedding is a novel mechanism for PMN detachment. First, the cleavage of CD11b was observed in both in vitro and in vivo migration systems, suggesting that the shedding is a specific event. This conclusion was strengthened by our identification of 3 leukocyte serprocidins as the candidate enzymes that cleave CD11b during PMN detachment and subsequent migration. The specific cleavage of CD11b before PMN detachment was also supported by our identification of the cleavage sites of the 3 leukocyte serprocidins on purified, functionally active CD11b/CD18. Second, blocking CD11b cleavage by specific inhibitor/antibody against the 3 serprocidins inhibited PMN migration across the epithelial monolayers (Figure 3). Third, the in vitro PMN detachment assays directly demonstrated that the cleavage of CD11b is involved in the release of adhered PMNs from immobilized fibrinogen.
Our finding of CD11b shedding during PMN detachment and subsequent migration provides another example that the shedding of adhesive molecules by leukocyte proteases is an important mechanism for leukocyte migration across endothelia, epithelia and tissue matrices. Recently, Evans et al15 reported the shedding of LFA-1 during leukocyte transmigration; however, the molecular basis of this event, including the candidate enzyme for integrin cleavage and the correlation between integrin cleavage and leukocyte detachment, remains unknown. Interestingly, the CD18 subunit of β2 integrin from macrophages was cleaved by MMP-9,33 although MMPs were not involved in CD11b shedding (Figure 5D). These results indicate that various enzymatic cleavage machineries modulate integrin shedding during leukocyte inflammatory responses. Previous studies have demonstrated the potential roles of the soluble ectodomains of integrins in the regulation of cellular adhesion and migration.15,47 Our data also indicate that the soluble CD11b ectodomain maintains its ligand-binding activity because it consists of the intact I-domain that is recognized by specific anti-CD11b antibodies. Thus, the shed CD11b ectodomain may contribute to the regulation of leukocyte adhesion and transmigration through competition for the ligand binding of endogenous CD11b/CD18.
The role of leukocyte serprocidins in modulating CD11b shedding during PMN migration
The identification of NE, NPR-3 and NCG as the major candidates for cleaving CD11b during PMN detachment and subsequent migration provides a novel mechanism for PMN transmigration. A direct cleavage assay using purified, functionally active CD11b/CD18 further confirmed the specific cleavage of CD11b by neutrophil serprocidins (Figures 5–6). The finding that NE, NCG and NPR-3 can all contribute to CD11b shedding and PMN detachment may also provide clues that explain the controversial reports18-20,22-26 on the roles of serprocidins in modulating PMN recruitment. Because each of the 3 serprocidins can effectively cleave CD11b, the combination of NE, NPR-3, and NCG forms a highly efficient mechanism for the rapid shedding of neutrophil CD11b during cell detachment and subsequent migration. In other words, PMNs have 3 serine proteases and all of them can cleave CD11b. Thus, the knockout of an individual protease would likely not completely prevent PMN transmigration. However, our results suggest that PMN transmigration would be completely abolished if all 3 serprocidins were inhibited. Indeed, in beige mice lacking multiple serine proteases (NE and NCG), PMNs have a profound chemotactic response defect.
The surface expression of PMN serprocidins in response to chemoattractants also suggests their potential roles in the cleavage of CD11b (supplemental Figure 2). NE was concentrated at one end of the migrating PMN (likely the uropod) where it was largely colocalized with CD11b/CD18. This observation is consistent with a previous observation of cell surface-bound NE and NCG on human PMNs,48 and supports the notion that NE was involved in the cleavage of the CD11b at the uropod where PMNs detach.
In the present study, we also identified the cleavage sites of NE, NPR-3 and NCG on purified, functionally active CD11b/CD18 (Figure 5). The 2 cleavage sites on CD11b by 3 PMN serprocidins are all located membrane proximally and separated by a single amino acid. The exact cleavage site of CD11b by endogenous neutrophil serprocidins during the cell transmigration, however, remains unknown. Although the cleavage site(s) on endogenous CD11b during PMN detachment and subsequent transmigration still need to be determined, several pieces of evidence indicate that the cleavage site identified using purified CD11b/CD18 may be identical to that of CD11b cleavage during a bona fide PMN transmigration: (1) The CD11b cleavage patterns in purified CD11b/CD18 (Figure 5) and endogenous CD11b/CD18 during PMN migration (Figure 1) or detachment (Figure 4) are almost identical; (2) The combination of the inhibitors specific to the 3 serprocidins completely blocked CD11b shedding and PMN migration, suggesting that the 3 serprocidins are the major candidates that cleave CD11b; and (3) A synthetic peptide matching the CD11b cleavage site sequence effectively competed for serprocidin binding of CD11b/CD18 and inhibited PMN migration (Figure 6).
Our results also show that cleavage of CD11b off PMN surfaces is likely dependent on CD11b/CD18-mediated cell adhesion. The binding assay showed that NE binds strongly to functionally active CD11b (Figure 5C), which is consistent with the observation that CD11b shedding is correlated with the PMN adhesion mediated by interactions of CD11b/CD18 with immobilized FBG (supplemental Figure 1).
In conclusion, the present study provides the first evidence that PMN CD11b extracellular domain containing the ligand-binding region is specifically cleaved by 3 leukocyte serprocidins (NE, NPR-3 and NCG) during PMN detachment and subsequent migration. The specific cleavage of CD11b serves as a novel mechanism for PMN detachment. The identification of the critical role of CD11b shedding by the 3 leukocyte serprocidins in the modulation of PMN detachment and transmigration provides potential effective therapeutic targets that aim to control and attenuate leukocyte inflammatory responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the excellent protein chemistry work by Drs Donghai Li (Nanjing University, Nanjing, China) and Jan Pohl (Emory University, Atlanta, GA).
This work was supported fully by American Heart Association grant-in-aid (0565251B, Y.L.), National Institutes of Health R21 grant (AI073622, Y.L.), American Heart Association National Scientist Development Grant (K.Z.), and the National Natural Science Foundation of China (30871019 and 30988003, K.Z.; 90813035 and 30890044, C.Y.Z.). The initial work of this study was performed at Dr Charles A. Parkos's laboratory at Emory University.
National Institutes of Health
Authorship
Contribution: K.Z. and Y.L. designed the research and analyzed data; K.Z. drafted the manuscript; K.Z., Y.-L.G., L.-M.L., and Z.B. performed research and analyzed data; and C.-Y.Z. contributed vital new reagents and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Ke Zen, PhD, School of Life Sciences, Nanjing University, Hankou Rd 22, Nanjing, Jiangsu 210093, China; e-mail: kzen@nju.edu.cn.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal