Abstract
An important role for natural killer (NK) cells in the regulation of T-cell responses is emerging, although the receptor pairs regulating the NK–T-cell interaction have still not been identified. We found that superantigen-stimulated T cells express Nectin-2 (CD112) and poliovirus receptor (PVR; CD155), the ligands of the activating NK receptor DNAX accessory molecule-1 (DNAM-1; CD226). Interestingly, only PVR was present at the T cell surface, particularly on cells in the S and G2/M phases of the cell cycle. The up-regulation of PVR expression involves DNA-damage response (DDR)–dependent pathways, because we found that pharmacologic inhibition of ATM and ATR kinases reduced PVR expression and that PVR was almost exclusively induced on cells expressing the DDR marker γH2AX. Oxidative stress contributed to DDR activation, and our results showed impaired PVR levels in the presence of the reactive oxygen species (ROS) scavenger N-acetyl-cysteine (NAC), being monocytes the main ROS source needed for optimal PVR expression on activated T cells. Interestingly, in accordance with ligand expression, NK cells lysed allogeneic proliferating more efficiently than nonproliferating T lymphocytes, with a mechanism requiring the cooperation between DNAM-1 and NKG2D. These results could contribute to unraveling the role of NK cells in the down-regulation of T-cell responses in physiologic and pathologic processes such as autoimmunity or GVHD.
Introduction
In addition to their role in providing antitumor and antiviral immunity,1 natural killer (NK) cells are also able to regulate the T-cell arm of the adaptive immune response by secreting different cytokines and chemokines.2 Moreover, several studies have provided evidence of cognate cell-cell interactions between NK cells and various leukocyte types, including dendritic cells (DCs) and B and T lymphocytes.3-5
Although NK cells have been thought to mainly promote adaptive immune responses, recent in vivo studies suggest that they can also restrain T cell–mediated immune responses. Therefore, the depletion of NK cells results in enhanced T-cell proliferation and effector functions during murine cytomegalovirus infection6 and in an antitumor response against lymphoma cells.7 Conversely, several studies have indicated that the depletion of NK cells is associated with increased severity of autoimmune diseases. In fact, NK cell–depleted mice develop a more severe form of experimental autoimmune encephalomyelitis,8 and NK-cell–mediated down-regulation of autoreactive cytotoxic T lymphocytes has been shown to have a protective role in type 1 diabetes.9 These findings suggest that NK cells may be crucial for terminating T cell–mediated responses and for preventing inappropriate T-cell activation and effector functions leading to the development of autoimmune diseases.
NK cell–mediated attenuation of T-cell responses can involve several mechanisms, including the production of inhibitory cytokines (eg, TGF-β and IL-10)10,11 and killing of DCs and/or activated T cells.4,12,13 In regard to the NK cell–mediated killing of T cells, IL-2–activated mouse and human NK cells recognize and lyse T-cell blasts in a perforin-dependent manner through the activating receptor NKG2D.4,12 Interestingly, the results of our previous study indicated that Ag stimulation of human T cells was sufficient to induce the surface expression of the NKG2D ligands (NKG2DLs) MHC class I–related chain A (MICA), MICB, and UL16-binding proteins 1-3 (ULBP1-3).4,14 To date, little is known about the existence of additional receptor-ligand interactions that might contribute to the NK cell–mediated recognition of T lymphocytes.
DNAX accessory molecule-1 (DNAM-1) is an activating receptor belonging to the Ig superfamily that is constitutively expressed by most NK cells, T cells, macrophages, and DCs.15,16 DNAM-1 interacts with lymphocyte function–associated antigen 1 (LFA-1), and this association is required for its functional activity on both NK and cytotoxic T cells.17 Ligands for DNAM-1 (DNAM-1Ls) include Nectin-2 and poliovirus receptor (PVR), which belong to the Nectin/Nectin-like family of adhesion molecules.18 DNAM1Ls are often expressed by tumor cells and can activate or enhance tumor cell lysis in vitro.15,18 Recent studies have reported that they can also be expressed by monocytes, DCs, and phytohemagglutinin (PHA)–stimulated CD4+ T lymphocytes.19,20
Little information is available about the molecular mechanisms regulating DNAM-1L expression. We have previously shown that DNAM-1Ls can be up-regulated on multiple myeloma cells in response to genotoxic stress through the activation of the DNA-damage response (DDR).21 DDR is initiated by 2 protein kinases: ATM (Ataxia telangiectasia mutated) and ATR (ATM- and RAD3-related). Both kinases generate a signaling cascade through the phosphorylation of several substrates, including the histone H2AX on Ser139 (γH2AX), which is widely used as a marker of DDR activation.22 Several studies have indicated that DDR can also be activated during physiologic processes such as mitosis23 and after lipopolysaccharide stimulation in macrophages.24
Among the different forms of stress leading to up-regulation of the ligands for activating NK-cell receptors, little is known about the role played by oxidative stress; however, much evidence indicates its involvement in the induction of DDR.25 A recent study showed that ATM kinase can be directly activated by ROS.26 Enhanced expression of MICA and MICB has been found on human colon carcinoma cells27 and on normal airway epithelial cells28 exposed to H2O2, and analyses of MICA/B genes also revealed a minimal core promoter that directs oxidative stress–induced transcriptional activation.29 The PVR promoter also contains a consensus sequence for nuclear respiratory factor-1 (Nrf-1) that is regulated by ROS.30
In the present study, we investigated the expression and function of DNAM-1Ls on human T cells in response to superantigen stimulation. We also examined signaling events responsible for the induction of PVR on activated T cells, showing that DDR activation and oxidative stress contribute to the regulation of PVR expression. Our results establish that PVR induction is related to cell division and is associated with γH2AX expression on activated T lymphocytes in a ROS-dependent manner. Moreover, we show that the DNAM-1/DNAM-1L axis participates in the NK-cell–mediated recognition and lysis of proliferating T cells.
Methods
Antibodies and reagents
The following mAbs were used: anti-NKG2D (MAB149810) and anti-MICB (MAB236511) from R&D Systems; anti-MICA (M673) from Amgen; anti–CD48/PE (TU145), anti–Nectin-2 (R2.525), anti–human leukocyte antigen I (HLA-I; W6/32), and anti-CD56 (C318) from Becton Dickinson; anti–DNAM-1 (DX11) from Serotec; anti–CD3/allophycocyanin (HIT3a), anti–CD3/FITC (HIT3a), anti–CD3/PECy5 (HIT3a), anti–CD8/PECy5 (HIT8a), anti–CD25/FITC (BC96), anti–CD25/allophycocyanin (BC96), anti–CD14 (HCD14), anti–CD14/FITC (HCD14), anti–CD14/allophycocyanin (HCD14), mouse control IgG, goat-anti–mouse (GAM)/PE (Poly4053), and GAM/allophycocyanin (Poly4053) from BioLegend; anti–β-actin from Sigma-Aldrich; GAM/HRP from Bio-Rad; anti-γH2AX (JBW301) from Millipore; anti–γH2AX/Alexa Fluor 647 (JBW301) from Cell Signaling Technology; anti-PVR (SKII.4) kindly provided by Dr M. Colonna (Washington University, St Louis, MO); and anti–LFA-1 (TS1/18) kindly provided by Dr F. Sanchez-Madrid (La Princesa Hospital, University of Madrid, Spain). Other reagents used were: formaldehyde, CFSE, Staphylococcus aureus enterotoxin B (SEB), propidium iodide (PI), caffeine, KU-55933, N-acetyl-cysteine (NAC), and 7-amino actinomycin D (7-AAD) from Sigma-Aldrich; human recombinant IL-2 from Peprotech; and PHA from Biochrom.
Cell stimulation and purification
PBMCs were obtained by Ficoll separation of peripheral blood samples from healthy donors and plated at a concentration of 1.5 × 106 cells/mL in the presence of SEB (100 ng/mL) or PHA (1 μg/mL). Cells were labeled with CFSE at day 0, as described previously.4 Where indicated, 48-hour SEB-stimulated cells were treated with caffeine (5mM), KU-55933 (30μM), NAC (5mM), or H2O2 (100μM), and harvested after an additional 18 hours. In some experiments, T cells were negatively isolated or monocytes were depleted from total PBMCs before stimulation using magnetic beads (Dynal; Invitrogen) according to the manufacturer's instructions.
Immunofluorescence and FACS analysis
CFSE-labeled PBMCs were stained with specific mAbs, followed by GAM/PE. Cells were then incubated with normal mouse IgG, followed by anti–CD3/allophycocyanin and anti–CD8/PECy5 mAbs or by anti–CD3/PECy5 and anti–CD25/allophycocyanin mAbs, and finally analyzed by flow cytometry on an FACSCalibur (Becton Dickinson). Proliferating cells were identified by gating the CFSElow population. The median fluorescence intensity (MFI) of the control isotype IgG was always subtracted from the MFI relative to each molecule. For intracellular staining, PBMCs were fixed in 2% paraformaldehyde (PFA) and permeabilized with 0.5% saponin. After washing, staining was performed as described. To simultaneously determine cell-cycle progression and PVR expression, cells were stained with anti-PVR followed by GAM/allophycocyanin and then incubated with normal mouse IgG followed by anti–CD3/FITC. Cells were fixed and permealized with 30% methanol plus 0.4% PFA in PBS for 15 minutes at 4°C, washed, left overnight at 4°C, and then incubated in 0.05% Tween plus 1% PFA in PBS for 1 hour at room temperature, washed, and stained with PI. In some experiments, CFSE-labeled PBMCs were stained with specific mAbs followed by GAM/PE. After washing, cells were fixed with 1% formaldehyde, permeabilized with 70% ethanol, and incubated with anti–γH2AX/Alexa Fluor 647 mAb. In some experiments, CFSE-labeled PBMCs were fixed with 1% formaldehyde, permeabilized with 70% ethanol, and then incubated with anti-γH2AX mAb followed by GAM/PE.
SDS-PAGE and Western blot
Highly purified T cells were lysed in a buffer containing 1% NP-40, 20mM Tris-HCl, pH 7.6, 140mM NaCl, 10% glycerol, 2.6mM MgCl2, 2.6mM CaCl2, 10mM NaF, 1mM Na3VO4, and protease inhibitors. Lysates were resolved by 10% SDS-PAGE and transferred to nitrocellulose membranes (Millipore). Membranes were blocked with 5% milk and probed with specific mAbs. Immunoreactivity was revealed using an enhanced chemiluminescence kit (Amersham).
RT-PCR
One microgram of total RNA was isolated using TRIzol reagent (Invitrogen) and used for cDNA first-strand synthesis in a 25-μL reaction volume; 1 μL of the resulting cDNA was used in a 25-μL PCR reaction in the presence of FastStart Taq DNA polymerase (Roche). Forward and reverse primers were: PVR, 5′-GAGGTGACGCATGTGTCACAG-3′, 5′-TCTTGCCGTCCACCTGGCTTG-3′; Nectin-2, 5′-GTCCTTCGTCTCTGCCAAGCA-3′, 5′-CACTGCGTGGATGACCAGCTG-3′; and GAPDH, 5′-ACCACAGTCCATGCCATCAC-3′, 5′-TCCACCACCCTGTTGCTGTA-3′. PCR conditions were as follows: 94°C for 50 seconds, 58°C for 50 seconds, and 72°C for 50 seconds for 28-32 cycles.
Alkaline comet assay
PBMCs (105 untreated or SEB stimulated for 3 days) were combined with 0.7% low-melting agarose, plated onto microscope slides previously spread with 1% normal melting agarose, and lysed overnight at 4°C in lysis buffer (100mM Na2EDTA, 2.5 M NaCl, 10mM Tris-HCl, 10% DMSO, and 1% Triton X-100). Slides were then incubated for 20 minutes in the presence of alkaline running buffer (300mM NaOH and 1mM Na2EDTA, pH > 10), electrophoresed at 20 V, 300 mA at 4°C for 20 minutes, and neutralized with 300mM sodium acetate, 90% ethanol for 30 minutes at room temperature. After staining with ethidium bromide, cells were scored using an Apotome microscope Observer Z.1 (Zeiss) equipped with a 40× objective. At least 100 randomly selected cells per sample were analyzed with CometScore (http://autocomet.com).
Cytotoxicity assay
A standard 51Cr-release assay was performed using long-term cultured NK cells activated for 18 hours with IL-2 (200 IU/mL) as effectors and autologous or allogeneic PBMCs stimulated for 3 days with SEB as targets.4 In some experiments, a flow cytometric cytotoxicity assay using CFSE-labeled, SEB-activated PBMCs as targets and IL-2–activated allogeneic NK cells as effectors was performed. Briefly, effector and target cells were incubated at different E:T ratios for 4 hours at 37°C and labeled with the intercalating DNA dye 7-AAD (5 μg/mL) for 20 minutes. Cells were washed, fixed in 1% PFA, and analyzed. Where indicated, effector cells were incubated for 20 minutes with blocking antibodies prior to incubation with the target cells. Cytotoxicity was also expressed as lytic units, which are defined as the number of effector cells necessary to lyse 15% of target cells.
Results
PVR and Nectin-2 are induced on Ag-activated T cells
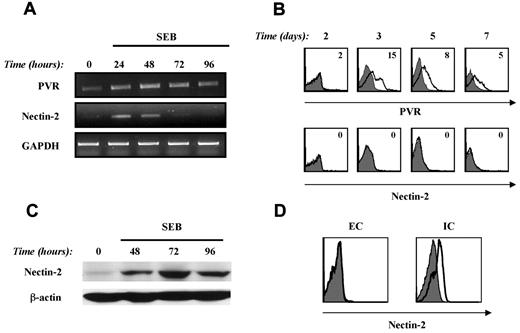
To determine if the DNAM1/DNAM1L interaction could play a role in the cross-talk between NK and T cells, we first investigated the expression of PVR and Nectin-2 on activated T cells. First, the expression of DNAM-1L mRNA was evaluated by RT-PCR on T cells purified from SEB-stimulated PBMCs. We found basal levels of PVR mRNA that were up-regulated 24 hours after stimulation and persisted up to 96 hours, whereas Nectin-2 mRNA levels, also evident at 24 hours, declined at 48 hours (Figure 1A).
PVR and Nectin-2 are induced on activated T cells in response to SEB stimulation. (A) PBMCs were stimulated with SEB (100 ng/mL) at different times, T lymphocytes were purified by immunomagnetic positive selection, and total RNA was isolated. RT-PCR was performed as described in “RT-PCR.” One representative donor of the 4 analyzed is shown. (B) PVR and Nectin-2 expression was evaluated by performing immunofluorescence and cytofluorimetric analysis by gating CD3+ T cells on SEB-stimulated PBMCs at days 2, 3, 5, and 7. MFI of isotypic control mAb was subtracted from PVR or Nectin-2 MFI. Time-course analysis was performed on 4 different donors and the average of PVR MFI ± SD was: day 0: 0 ± 0; day 2: 2 ± 0; day 3: 14 ± 4; day 5: 11 ± 4; and day 7: 5 ± 1. A representative donor is shown. (C) PBMCs were stimulated with SEB and T cells were purified as described in panel A. Western-blot analysis was performed on total cell lysate using an anti–Nectin-2 mAb. Protein loading was normalized using β-actin. Lysates from the K562 and BAF3 cell lines were used as positive and negative controls, respectively (data not shown). (D) PBMCs were stimulated with SEB for 3 days and then stained with an isotypic control mAb (filled histogram) or Nectin-2 mAb (open histogram). Extracellular (EC) and intracellular (IC) staining was performed as described in “Methods.” Nectin-2 expression was analyzed by gating the CD3+ T-cell population.
PVR and Nectin-2 are induced on activated T cells in response to SEB stimulation. (A) PBMCs were stimulated with SEB (100 ng/mL) at different times, T lymphocytes were purified by immunomagnetic positive selection, and total RNA was isolated. RT-PCR was performed as described in “RT-PCR.” One representative donor of the 4 analyzed is shown. (B) PVR and Nectin-2 expression was evaluated by performing immunofluorescence and cytofluorimetric analysis by gating CD3+ T cells on SEB-stimulated PBMCs at days 2, 3, 5, and 7. MFI of isotypic control mAb was subtracted from PVR or Nectin-2 MFI. Time-course analysis was performed on 4 different donors and the average of PVR MFI ± SD was: day 0: 0 ± 0; day 2: 2 ± 0; day 3: 14 ± 4; day 5: 11 ± 4; and day 7: 5 ± 1. A representative donor is shown. (C) PBMCs were stimulated with SEB and T cells were purified as described in panel A. Western-blot analysis was performed on total cell lysate using an anti–Nectin-2 mAb. Protein loading was normalized using β-actin. Lysates from the K562 and BAF3 cell lines were used as positive and negative controls, respectively (data not shown). (D) PBMCs were stimulated with SEB for 3 days and then stained with an isotypic control mAb (filled histogram) or Nectin-2 mAb (open histogram). Extracellular (EC) and intracellular (IC) staining was performed as described in “Methods.” Nectin-2 expression was analyzed by gating the CD3+ T-cell population.
We then analyzed the cell-surface expression of DNAM-1Ls by gating CD3+ cells from PBMCs either unstimulated or stimulated with SEB for several days. We did not detect any DNAM-1Ls on resting T cells (data not shown), whereas PVR but not Nectin-2 was expressed on the surface of activated T lymphocytes (Figure 1B). PVR expression peaked at day 3, declined at day 5, and was confined to a fraction of stimulated T cells. No major differences in PVR expression were detected between activated CD4+ and CD8+ T cells (data not shown). Similar results were obtained in response to PHA stimulation (data not shown). Because PVR transcripts, but not cell-surface proteins, were already detected in T cells under basal conditions and were increased at 24 and 48 hours after stimulation, we also performed intracellular staining for PVR on unstimulated and SEB-activated T cells at different times. We found very low basal levels of total PVR protein that were strongly up-regulated at 48 hours and persisted at 72 hours after stimulation (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Our failure to detect Nectin-2 surface expression on activated T cells was not attributable to the mAb used because this antibody revealed Nectin-2 on the surface of 293T cells used as a control (data not shown). We then performed Western blot analysis on highly purified SEB-stimulated T cells, and a band corresponding to the Nectin-2 protein was detected at 48 hours of stimulation and persisted up to 96 hours (Figure 1C). No Nectin-2 expression was detected on resting T cells. Moreover, immunostaining and flow cytometric analysis of permeabilized PBMCs after a 3-day SEB stimulation showed intracellular expression of Nectin-2 on activated T lymphocytes (Figure 1D).
These results demonstrate that PVR and Nectin-2 are induced on T cells in response to Ag stimulation at both the mRNA and the protein level. However, only PVR is expressed on the cell surface, suggesting that distinct posttranslational mechanisms regulate the expression of different DNAM-1Ls on activated T cells.
PVR is mainly expressed on T cells proliferating in response to Ag stimulation
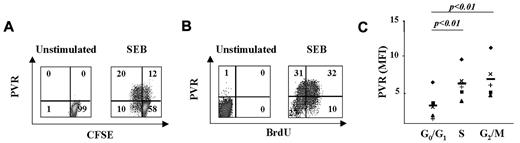
We have previously shown that T lymphocytes proliferating in response to Ag stimulation preferentially express NKG2DLs.4,14 Therefore, to analyze whether T lymphocytes also display high levels of DNAM-1Ls, we used immunofluorescence and FACS analysis to evaluate PVR expression on CFSE-labeled PBMCs after SEB stimulation. At day 3, PVR was expressed on the majority (∼70%) of SEB-stimulated T lymphocytes that had divided (CFSElow), and only on a small fraction of nondividing cells (CFSEhigh) (Figure 2A and supplemental Figure 2A).
PVR expression on activated T cells is associated with progression to the S and G2/M phases of the cell cycle. (A) CFSE-labeled PBMCs were activated or not with SEB for 3 days and PVR expression was analyzed by gating CD3+ T cells. We used CFSE fluorescence intensity to divide T cells into 2 populations: those that had undergone at least one cell division (CFSElow) and those that had not divided (CFSEhigh). The percentage of cells in each quadrant is reported. One representative donor of the 5 tested is shown. (B) Unstimulated or 3-day SEB-stimulated PBMCs were labeled with BrdU for 1 hour and then stained with anti-PVR mAb plus GAM/PE and anti-BrdU/FITC mAb. The percentage of cells in each quadrant is displayed. One representative donor of the 6 tested is shown. (C) PBMCs were stimulated with SEB for 3 days, stained with anti-PVR mAb plus GAM/allophycocyanin followed by anti–CD3/FITC, and then labeled with PI. CD3+ T cells were gated in the different phases of the cell cycle according to DNA content, and PVR expression was analyzed. The MFI value of the isotypic control mAb was subtracted from the MFI values of PVR. A summary of the MFI mean value ± SD from 5 donors is shown. Statistical analysis was with the Student paired t test.
PVR expression on activated T cells is associated with progression to the S and G2/M phases of the cell cycle. (A) CFSE-labeled PBMCs were activated or not with SEB for 3 days and PVR expression was analyzed by gating CD3+ T cells. We used CFSE fluorescence intensity to divide T cells into 2 populations: those that had undergone at least one cell division (CFSElow) and those that had not divided (CFSEhigh). The percentage of cells in each quadrant is reported. One representative donor of the 5 tested is shown. (B) Unstimulated or 3-day SEB-stimulated PBMCs were labeled with BrdU for 1 hour and then stained with anti-PVR mAb plus GAM/PE and anti-BrdU/FITC mAb. The percentage of cells in each quadrant is displayed. One representative donor of the 6 tested is shown. (C) PBMCs were stimulated with SEB for 3 days, stained with anti-PVR mAb plus GAM/allophycocyanin followed by anti–CD3/FITC, and then labeled with PI. CD3+ T cells were gated in the different phases of the cell cycle according to DNA content, and PVR expression was analyzed. The MFI value of the isotypic control mAb was subtracted from the MFI values of PVR. A summary of the MFI mean value ± SD from 5 donors is shown. Statistical analysis was with the Student paired t test.
We also used the bromodeoxyuridine (BrdU) incorporation assay on PBMCs pulsed for 1 hour at day 3 after SEB stimulation to measure PVR expression on cells that were in or entering into the S phase of the cell cycle. We found that approximately 40% of T cells incorporated BrdU and that the majority expressed PVR (Figure 2B and supplemental Figure 2B). Of note, intracellular PVR expression was mainly restricted to BrdU+ T lymphocytes and was already evident after 48 hours of stimulation, before detection on the cell surface (supplemental Figure 3).
The preferential expression of PVR on CFSElow and BrdU+ cells prompted us to further analyze its distribution on T cells during the different phases of cell cycle (Figure 2C). T cells in the S and G2/M phases expressed significantly higher levels of PVR compared with cells in the G0/G1 phases. No changes in HLA-I expression (used as a control) were observed during cell-cycle progression (data not shown).
These results indicate that PVR is mainly expressed on T cells that are in the S and G2/M phases of the cell cycle, suggesting that signaling pathways associated with Ag-induced T-cell proliferation might be implicated in the regulation of PVR expression.
PVR up-regulation on activated T cells partially depends on ATM/ATR kinases and activation of the DDR
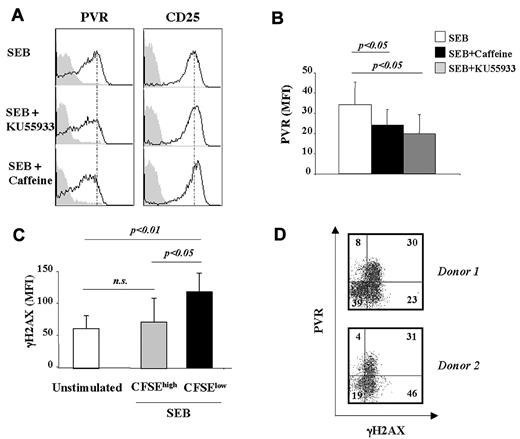
We have previously shown that up-regulation of PVR on multiple myeloma cells triggered by genotoxic stress is dependent on the activation of ATM/ATR protein kinases.21 Moreover, increasing evidence shows that the ATM/ATR–initiated signaling cascade may also be activated during more physiologic processes, such as normal mitosis.23 Therefore, we evaluated whether the induction of PVR on T cells in response to SEB stimulation was dependent on the activation of DDR. Two different drugs were used initially: caffeine, an inhibitor of ATM/ATR catalytic activity, and KU-55933, which is specific for ATM. CFSE-labeled PBMCs were stimulated for 2 days with SEB, treated with caffeine or KU-55933 at doses previously chosen for their specific activity,4 and harvested after an additional 18 hours. PVR expression was then analyzed on CFSElow T cells. Both caffeine and KU-55933 treatment markedly inhibited PVR expression on T cells that underwent at least one cell division, suggesting the involvement of DDR activation in the up-regulation of PVR (Figure 3A-B). Neither the percentage of proliferating T cells nor the expression of CD25 used as a control was affected by inhibitor treatment (Figure 3A and data not shown).
PVR up-regulation is ATM/ATR dependent and is associated with enhanced H2AX phosphorylation. (A) CFSE-labeled PBMCs were stimulated with SEB. At day 2, cells were treated with caffeine (5mM) or KU-55933 (30μM). After an additional 18 hours, cells were stained with anti–CD3/PECy5, anti-PVR/PE (empty histogram), anti–CD25/allophycocyanin (empty histogram), or control isotypic Ig (filled histogram) and PVR and CD25 expression was analyzed on CD3+CFSElow T cells. One representative experiment of 3 is shown. (B) PBMCs were prepared as described in panel A. The MFI of isotypic control mAb was subtracted from PVR MFI. Data are represented as the mean values of MFI ± SD of PVR on CD3+CFSElow cells of 4 different donors. Statistical analysis was with the Student paired t test. (C) CFSE-labeled PBMCs were stimulated or not with SEB for 3 days and stained with anti–γH2AX mAb plus GAM/PE followed by anti–CD3/allophycocyanin mAb. γH2AX expression on SEB-stimulated T cells was analyzed on CFSElow and CFSEhigh populations. The mean values of MFI ± SD of 7 donors tested are shown. Statistical analysis was with the Student paired t test. (D) CFSE-labeled PBMCs were stimulated with SEB for 3 days and stained with anti-PVR mAb plus GAM/PE followed by anti–γH2AX/Alexa Fluor 647 mAb. The percentage of cells in each quadrant is reported. The 2 donors shown are representative of the 6 analyzed.
PVR up-regulation is ATM/ATR dependent and is associated with enhanced H2AX phosphorylation. (A) CFSE-labeled PBMCs were stimulated with SEB. At day 2, cells were treated with caffeine (5mM) or KU-55933 (30μM). After an additional 18 hours, cells were stained with anti–CD3/PECy5, anti-PVR/PE (empty histogram), anti–CD25/allophycocyanin (empty histogram), or control isotypic Ig (filled histogram) and PVR and CD25 expression was analyzed on CD3+CFSElow T cells. One representative experiment of 3 is shown. (B) PBMCs were prepared as described in panel A. The MFI of isotypic control mAb was subtracted from PVR MFI. Data are represented as the mean values of MFI ± SD of PVR on CD3+CFSElow cells of 4 different donors. Statistical analysis was with the Student paired t test. (C) CFSE-labeled PBMCs were stimulated or not with SEB for 3 days and stained with anti–γH2AX mAb plus GAM/PE followed by anti–CD3/allophycocyanin mAb. γH2AX expression on SEB-stimulated T cells was analyzed on CFSElow and CFSEhigh populations. The mean values of MFI ± SD of 7 donors tested are shown. Statistical analysis was with the Student paired t test. (D) CFSE-labeled PBMCs were stimulated with SEB for 3 days and stained with anti-PVR mAb plus GAM/PE followed by anti–γH2AX/Alexa Fluor 647 mAb. The percentage of cells in each quadrant is reported. The 2 donors shown are representative of the 6 analyzed.
We next examined whether the up-regulation of PVR on CFSElow cells was associated with increased expression of the ATM/ATR substrate γH2AX. CFSElow T cells expressed higher levels of γH2AX compared with undivided (CFSEhigh) or unstimulated T lymphocytes (Figure 3C), strongly indicating that DDR activation parallels T-cell proliferation. Remarkably, almost all PVR+ cells were also γH2AX+ (Figure 3D), whereas HLA-I molecules, which were used as a control, were equally distributed between γH2AX+ and γH2AX− cells (data not shown).
Because ATM/ATR activation and H2AX phosphorylation have been not exclusively described in the context of DDR,31 we verified the occurrence of DNA double- and single-strand breaks by performing the comet assay. As expected, we found that proliferating T cells stimulated for 3 days with SEB had a higher number of DNA strand breaks compared with resting T cells (supplemental Figure 4).
These data demonstrate that ATM/ATR–initiated DDR is activated in response to T-cell stimulation, and that these kinases cooperate in the regulation of PVR expression.
Oxidative stress plays an important role in DDR-dependent PVR expression on Ag-activated T cells
Based on the evidence showing that oxidative stress strongly induces the activation of DDR,25 we examined the ability of the antioxidant agent NAC, a glutathione precursor acting as a ROS scavenger, to interfere with DDR-mediated PVR induction on activated T cells in response to SEB stimulation.
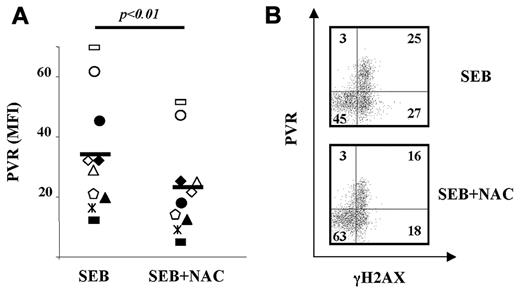
CFSE-labeled PBMCs were stimulated for 2 days and then NAC was added for an additional 18 hours. NAC treatment resulted in a significant reduction of PVR on CFSElow T lymphocytes from all donors tested (Figure 4A) without significantly affecting the percentage of CFSElow cells (data not shown), suggesting that ROS generation is involved in the up-regulation of PVR expression on proliferating T cells.
Oxidative stress is involved in PVR up-regulation on Ag-activated T cells. (A) CFSE-labeled PBMCs were activated with SEB. NAC (5mM) was added after 48 hours and left for an additional 18 hours. Cells were then stained as described in the legend to Figure 3A. The MFI of isotypic control mAb was subtracted from PVR MFI. Each of the 10 donors tested is represented by a different symbol. Statistical analysis was with the Student paired t test. (B) PBMCs were activated and treated as described in panel A and then stained as described in the legend to Figure 3B. The percentage of cells in each quadrant is displayed. The experiment shown is representative of the 6 performed.
Oxidative stress is involved in PVR up-regulation on Ag-activated T cells. (A) CFSE-labeled PBMCs were activated with SEB. NAC (5mM) was added after 48 hours and left for an additional 18 hours. Cells were then stained as described in the legend to Figure 3A. The MFI of isotypic control mAb was subtracted from PVR MFI. Each of the 10 donors tested is represented by a different symbol. Statistical analysis was with the Student paired t test. (B) PBMCs were activated and treated as described in panel A and then stained as described in the legend to Figure 3B. The percentage of cells in each quadrant is displayed. The experiment shown is representative of the 6 performed.
Interestingly, ROS-mediated regulation appears to be preferential for DNAM-1L, because NAC treatment had a marginal effect or no effect on the expression of the MICA and MICB NKG2DLs, which are also induced on activated T cells in an ATM/ATR–dependent manner4 (Table 1).
Effect of NAC treatment on MICA and MICB expression in T cells stimulated for 3 days with SEB
PBMCs were stimulated with SEB. After 48 hours, NAC was added to the cultures and left for an additional 18 hours. Cells were stained with specific mAbs plus GAM/PE and with anti–CD3/PECy5 mAb. NKG2DL expression was analyzed on CD3+CFSElow T cells. The MFI of isotypic control mAb was subtracted from the MFI of NKG2DL. The mean of MFI values of 6 different donors ± SD is shown. Statistical analysis was with the Student paired t test.
P < .05.
Not significant.
To investigate the relevance of ROS in the induction of PVR on SEB-stimulated T cells harboring a marker of DDR activation, we analyzed the effect of NAC on PVR and γH2AX coexpression on these cells. This treatment resulted in a marked reduction in the proportion of PVR- and γH2AX-coexpressing cells that paralleled the increased percentage of double-negative cells (Figure 4B).
These findings indicate that oxidative stress–dependent activation of DDR determines PVR up-regulation on T cells proliferating in response to Ag stimulation.
CD14+ cell–derived ROS contribute to PVR expression on activated T cells
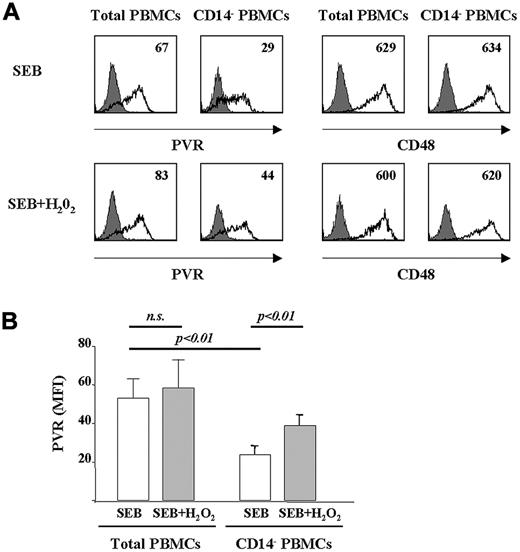
To understand the cellular source of the ROS responsible for PVR expression on activated T cells, we investigated the involvement of ROS produced by monocytes during Ag-induced PBMC stimulation. Total or CD14+ cell–depleted, CFSE-labeled PBMCs were stimulated with SEB for 3 days and PVR expression was analyzed on CFSElow T cells. PVR was strongly reduced on T cells from the monocyte-depleted PBMCs (Figure 5A). Decreased PVR expression was not attributable to the expected decreased number of proliferating T cells after monocyte depletion, because analysis was performed only on CFSElow T cells (ie, those that had undergone at least one cell division). Furthermore, the expression of other cell-surface receptors (ie, CD48 and CD25) was not affected by the removal of CD14+cells (Figure 5A and data not shown).
ROS produced by monocytes contribute to PVR expression on Ag-activated T cells. (A) CFSE-labeled PBMCs were immunodepleted or not depleted of CD14+ cells. Total PBMCs or CD14− PBMCs were stimulated with SEB for 2 days and treated or not with H2O2 (100μM). Staining and FACS analysis were performed after an additional 24 hours on CFSElow T cells, as described in the legend to Figure 3A. CD48 expression was analyzed as a control. Filled histogram shows the isotypic control mAb, and the black line shows PVR or CD48. PVR or CD48 MFI values subtracted from isotypic control mAb value are shown. (B) Mean values of MFI ± SD of PVR expression on total and CD14− PBMCs treated or not with H2O2 (100μM) from 3 different donors tested. Statistical analysis was with the Student paired t test.
ROS produced by monocytes contribute to PVR expression on Ag-activated T cells. (A) CFSE-labeled PBMCs were immunodepleted or not depleted of CD14+ cells. Total PBMCs or CD14− PBMCs were stimulated with SEB for 2 days and treated or not with H2O2 (100μM). Staining and FACS analysis were performed after an additional 24 hours on CFSElow T cells, as described in the legend to Figure 3A. CD48 expression was analyzed as a control. Filled histogram shows the isotypic control mAb, and the black line shows PVR or CD48. PVR or CD48 MFI values subtracted from isotypic control mAb value are shown. (B) Mean values of MFI ± SD of PVR expression on total and CD14− PBMCs treated or not with H2O2 (100μM) from 3 different donors tested. Statistical analysis was with the Student paired t test.
To further support the role of monocyte-derived ROS in the up-regulation of PVR on activated T cells, we examined the effect of H2O2 addition on SEB-stimulated, CD14+cell-depleted PBMC cell cultures. We found that the addition of exogenous ROS strongly increased PVR expression on T cells from CD14+ cell–depleted PBMCs, but not from total PBMC cultures (Figure 5A-B). Interestingly, the up-regulation of PVR induced by H2O2 was almost abrogated in the presence of KU-55933 and caffeine, providing evidence for a crucial link between ROS-dependent activation of DDR and induction of PVR (supplemental Figure 5). CD14+ cell–depleted PBMCs were also treated with NAC, which only slightly reduced PVR expression on CFSElow T cells (data not shown), suggesting a minor role for ROS derived from cells other than monocytes in PVR induction.
These results indicate that ROS, which are mostly produced by monocytes, play a critical role in the regulation of PVR expression through the activation of DDR on proliferating T cells.
DNAM-1/PVR interaction is involved in NK cell–mediated lysis of activated T cells
Our previous findings showed that the killing of activated T cells by autologous NK cells is dependent on the NKG2D/NKG2DL interaction, with only a minor contribution from other activating receptors.4 Therefore, we performed cytotoxicity assays to investigate the role of DNAM-1 in an autologous setting using activated T cells as targets and autologous polyclonal cultures of NK cells as effectors in the presence of neutralizing antibodies against DNAM-1 and NKG2D. As expected, NKG2D was the main receptor involved in the lysis, but we also observed a minor but significative contribution for DNAM-1 (supplemental Figure 6).
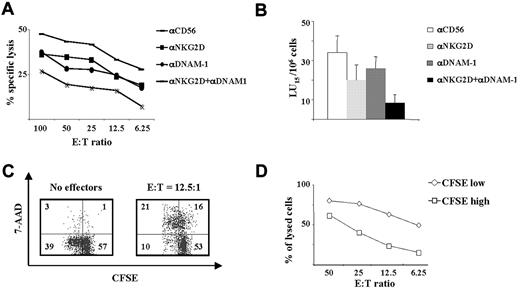
Based on the evidence that DNAM-1 has a major role in NK cell–mediated killing of allogeneic mature DCs, we examined whether this receptor could be also involved in the lysis of allogeneic activated T cells. We evaluated the role of DNAM-1/DNAM-1L and NKG2D/NKG2DL interactions in the NK cell–mediated lysis of allogeneic activated T cells by performing the assay in the presence of specific neutralizing mAbs alone or in combination. We observed that NK cell–mediated cytotoxicity was partially reduced by anti-NKG2D or anti-DNAM-1 mAbs when used alone, whereas when used in combination they markedly inhibited NK-cell cytotoxicity. These results indicate that NK-cell killing of activated allogeneic T lymphocytes depends on the cooperation between DNAM-1- and NKG2D-activating receptors (Figure 6A-B). Moreover, because PVR and NKG2DLs are mostly expressed on proliferating T cells, we investigated whether NK cells preferentially recognize and kill cells that have undergone at least one cell division. We performed a flow cytometry–based cytotoxicity assay using IL-2–activated NK cells as effectors, CFSE-labeled SEB-activated allogeneic PBMCs as targets, and 7-AAD to detect dead cells. Interestingly, consistent with the extent of NK-activating ligand expression, NK cells killed CFSElow T cells more efficiently than CFSEhigh T cells at all E:T ratios tested (Figure 6C).
DNAM-1 and NKG2D are both involved in the NK-cell–mediated killing of allogeneic activated T cells. (A) A classic 51Cr-based cytotoxicity assay was performed using IL-2–activated NK cells as effectors and 3-day SEB-activated allogeneic PBMCs as targets at the indicated E:T ratios. The assay was performed in the presence of neutralizing mAbs against NKG2D and/or DNAM-1 or against CD56 used as an isotype control. The data shown are representative of 1 of 6 donors tested. (B) Mean ± SE of lytic units (LU) calculated on 6 different donors. (C) NK cells, used as effectors, were incubated at 37°C with allogeneic, CFSE-labeled, 3-day SEB-stimulated PBMCs used as targets at an E:T ratio of 6.25:1, 12.5:1, 25:1, and 50:1. After 4 hours, cells were stained with 7-AAD, as described in “Immunofluorence and FACS analysis.” Analysis of target cells was performed by gating CFSE+ cells. An E:T ratio of 12.5:1 is shown. Numbers represent the percentage of cells in each quadrant. The experiment shown is representative of the 8 performed. (D) The percentage of lysed cells was analyzed by calculating the percentage of 7-AAD+ target cells in the CFSElow (◇) or CFSEhigh (□) populations. The donor analyzed is the same as that in panel C.
DNAM-1 and NKG2D are both involved in the NK-cell–mediated killing of allogeneic activated T cells. (A) A classic 51Cr-based cytotoxicity assay was performed using IL-2–activated NK cells as effectors and 3-day SEB-activated allogeneic PBMCs as targets at the indicated E:T ratios. The assay was performed in the presence of neutralizing mAbs against NKG2D and/or DNAM-1 or against CD56 used as an isotype control. The data shown are representative of 1 of 6 donors tested. (B) Mean ± SE of lytic units (LU) calculated on 6 different donors. (C) NK cells, used as effectors, were incubated at 37°C with allogeneic, CFSE-labeled, 3-day SEB-stimulated PBMCs used as targets at an E:T ratio of 6.25:1, 12.5:1, 25:1, and 50:1. After 4 hours, cells were stained with 7-AAD, as described in “Immunofluorence and FACS analysis.” Analysis of target cells was performed by gating CFSE+ cells. An E:T ratio of 12.5:1 is shown. Numbers represent the percentage of cells in each quadrant. The experiment shown is representative of the 8 performed. (D) The percentage of lysed cells was analyzed by calculating the percentage of 7-AAD+ target cells in the CFSElow (◇) or CFSEhigh (□) populations. The donor analyzed is the same as that in panel C.
These data show that NK cell–mediated killing of activated T lymphocytes is mainly directed against proliferating cells (ie, those expressing higher levels of ligands for NK cell–activating receptors).
Discussion
In the present study aimed at identifying the receptor/ligand pairs involved in the NK–T-cell cross-talk, we investigated the expression and function of the DNAM-1Ls PVR and Nectin-2 on Ag-activated T cells, as well as the mechanisms underlying their regulation. We showed that PVR expression was induced on the surface of stimulated T cells, whereas Nectin-2 was mostly retained at the intracellular level, showing that these 2 distinct DNAM-1Ls are differently regulated. This result on activated T cells, together with previous evidence describing the presence of Nectin-2 on the cell surface of other leukocytes (including mast cells,32 monocytes, and immature and mature myeloid DCs,19 ), are highly suggestive of a cell-specific posttranslational regulation of Nectin-2 expression.
Our findings also show that the expression of PVR on T lymphocytes is almost exclusively confined to cells that are in the S or G2/M phases of the cell cycle and that have undergone at least one division as measured by the loss of CFSE intensity. These findings are in accordance with previous reports describing a preferential expression of PVR on proliferating rat hepatocytes during liver regeneration and acute injury.33
To date, little is known about the signaling pathways underlying the regulation of DNAM-1L expression. Our results demonstrate that ROS-mediated ATM/ATR activation and initiation of DDR play a major role in the up-regulation of PVR on proliferating T cells. The involvement of ATM/ATR activation in the regulation of PVR expression was suggested by the ability of the pharmacologic inhibitors caffeine and KU-55933 to markedly reduce PVR levels. In addition, we have provided direct evidence showing that all PVR+ proliferating T cells also express higher levels of the ATM/ATR cellular substrate γH2AX. Finally, ATM/ATR activation triggered by Ag-stimulated T-cell proliferation was associated with DNA strand breaks, as shown with the comet assay.
Our results are consistent with previous reports showing that phosphorylation of ATM and its substrate H2AX are markedly enhanced after PHA stimulation of PBMCs.34 They are also in agreement with our previous findings showing a key role for ATM/ATR protein kinases in the up-regulation of PVR in multiple myeloma cells in response to treatment with genotoxic agents.21 Finally, our results further support the existence of a link between Ag-driven immune responses, such as the immunoglobulin class-switch recombination and somatic hypermutation involving the DDR machinery, and the induction of ligands for NK cell–activating receptors.35,36
The evidence that the PVR gene promoter contains a binding site for Nrf-1, a transcription factor regulated by oxidative stress,30 and that oxidative stress can activate DDR25 led us to reasonably hypothesize the involvement of ROS in the regulation of PVR expression. Interestingly, our data show that ROS scavenging significantly results in a reduced percentage of PVR+ γH2AX+ T cells, indicating that ROS generation contributes significantly to PVR regulation on proliferating T cells by activating ATM/ATR–dependent pathways. We also observed that NAC treatment did not affect MICB expression but did have a mild effect on MICA, suggesting that on activated T cells, oxidative stress preferentially leads to up-regulation of DNAM-1 rather than NKG2D ligands.
Accumulating evidence indicates that similar signaling pathways can lead to the expression of the ligands for different NK-activating receptors under both physiologic and stress conditions.4,14,21,33,37-39 We have recently reported enhanced expression of both DNAM-1 and NKG2D ligands on multiple myeloma cells in response to genotoxic stress in an ATM/ATR manner. However, there is also evidence demonstrating that different pathways can induce the ligands of distinct NK-activating receptors. Therefore, MICA and MICB, but not PVR, are up-regulated upon activation of the heat-shock response.40 In addition, the results of the present study clearly show that ROS generation plays a relevant role in the triggering of DNAM-1Ls on proliferating T cells, whereas it has only a minor contribution in NKG2DL up-regulation. Conversely, oxidative stress has been previously found to induce NKG2DL expression in other cell systems.27,28,41 These findings highlight the complexity of the molecular mechanisms leading to the induction of different ligands for distinct NK-activating receptors, and further support the notion that they may involve common or distinct pathways depending on the form of stress, the type of ligand, and the cellular context.
Our study also shows that monocytes are the main cellular source of ROS that regulate PVR expression during Ag-induced T-cell proliferation. Indeed, we observed reduced levels of PVR on proliferating T cells cultured in the context of CD14+ cell–depleted PBMCs, which were almost completely restored upon addition of H2O2. Another important piece of evidence linking ROS generation to ATM/ATR activation and PVR expression was that inhibitors of DDR prevented the increase of PVR induced by H2O2 treatment. These findings underline a novel role for monocytes in limiting T-cell responses. However, based on the evidence that T lymphocytes can also produce ROS in response to TCR triggering,42 we cannot exclude their possible contribution.
The expression of PVR and NKG2DLs on the majority of proliferating T lymphocytes opens the intriguing possibility that DNAM-1/DNAM-1L and NKG2D/NKG2DL interactions could cooperate in the NK cell–mediated regulation of T-cell activation and proliferative response. In fact, our results show that T cells undergoing at least one cell division are more susceptible to NK-cell lysis. Similarly, NK cells have been found to kill MHC class I–deficient T cells stimulated with concanavalin A more efficiently, strongly suggesting that the increased recognition by NK cells of activated and proliferating T cells is associated with the increased expression of ligands for activating receptors.43,44 In addition, it was recently reported in a variety of cell lines that cells in mitosis are more susceptible to NK killing compared with cells in other phases of the cell cycle, with a major role for natural cytotoxicity receptors (ie, NKp44 and NKp46) and only a minor contribution for NKG2D.45
Our results demonstrate that unlike in the autologous setting, where NKG2D plays the major role in NK cell–mediated cytotoxicity against activated T cells,4 both receptors strongly contribute to the lysis of allogeneic target cells. Similarly, DNAM-1 is preferentially involved in NK-cell lysis of allogeneic mature DCs.19 Therefore, the engagement of NK-inhibitory receptors by self-HLA class I molecules may protect activated T cells from DNAM-1–mediated NK-cell lysis. It is conceivable that killer cell immunoglobulin–like receptor–dependent reduction of LFA-1 density at the effector-target cell contact area46 may impair the DNAM-1–signaling ability that relies on the association with LFA-1.17
Because our data also showed a minor contribution of DNAM-1 in the autologous setting, it is possible that this receptor may play a more prominent role when NKG2D expression and/or functions are suboptimal. In fact, DNAM-1–dependent cytotoxic activity against several tumor cell lines has been observed only when the NKG2D receptor was masked.18 We can envisage that in all of those circumstances in which NKG2D is down-modulated by its ligands, released either by activated T cells in the course of an immune response14 or by other cell types under pathologic conditions,47 DNAM-1 could emerge as a dominant receptor.
Our findings support the idea that NK cells can prevent GVHD by eliminating activated T cells through the activity of DNAM-1– and NKG2D-activating receptors. A protective role of NK cells in GVHD was demonstrated several years ago and was attributed to the ability of NK cells to kill recipient APCs.48 Recent studies also underline the importance of direct NK–T-cell cross-talk in impairing the GVH response. In a murine model of allogeneic bone marrow transplantation, it was shown that NK and T cells from the graft home to the same host tissues and that NK cells counteract the activation and proliferation of donor T cells by direct killing through a mechanism partially dependent on NKG2D.49 Similarly, Rivas et al reported a role for NKG2D/NKG2DL interactions in the perforin-independent NK cell–mediated prevention of CD4+ T cell–induced GVHD.50
Our observations that in addition to NKG2DLs, proliferating T cells can also express PVR and become susceptible to DNAM-1–dependent NK-cell lysis in an allogeneic setting add another level of complexity to the NK–T-cell dialog and support the hypothesis that the DNAM-1 receptor can also contribute significantly to the elimination of donor T cells and to GVHD prevention.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Maria Rosaria D'Errico for expertise with the comet assay, Francesco Spallotta and Chiara Cencioni for their invaluable help, Rossella Paolini and Rosa Molfetta for microscope analysis, and Dina Milana for technical assistance.
This work was supported by grants from the Italian Association for Cancer Research (AIRC; Milan, Italy), from the Italian Ministry of University and Research (MIUR; Rome, Italy), from the Italian Ministry of Public Health (GR-683938), and from the Center of Excellence (BEMM; Rome, Italy).
Authorship
Contribution: M.A. and A.Z. designed research, performed experiments, and wrote the paper; C.C. designed research and performed experiments; F.C., A.S., and M.L.I. performed experiments; and A.S. designed research and contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alessandra Zingoni, Department of Molecular Medicine, University of Rome Sapienza, Policlinico Umberto I, Viale Regina Elena, 324, 00161 Rome, Italy; e-mail: alessandra.zingoni@uniroma1.it.
References
Author notes
M.A. and A.Z. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal