Abstract
GVHD and tumor relapse are fundamental problems in allogeneic HSCT. Recent research has linked neovascularization to GVHD, tumor growth, and graft-versus-tumor (GVT) activity. Damage of the endothelium by the conditioning regimen provides the initiation stimulus for recruitment of donor-derived endothelial cells and their progenitors. During the early inflammatory phase of GVHD there is considerable neovascularization facilitating migration of inflammatory cells to target organs. In the course of GVHD, however, the vasculature itself becomes a target of alloreactive donor T cells. As a consequence, later stages of GVHD are characterized by fibrosis and rarefaction of blood vessels. Importantly, the inhibition of tumor-neovascularization by activated donor T cells that release antiangiogenic substances contributes to GVT and may be enhanced by pharmacologic inhibition of neovascularization. Furthermore, the therapeutic inhibition of neovascularization may improve immunotherapy for cancer by enhancing leukocyte infiltration in tumor tissue because of normalization of tumor vessels and stimulation of leukocyte–vessel wall interactions. These insights identify important mechanisms underlining the importance of neovascularization for allogeneic immune responses and move therapeutic approaches targeting neovascularization into the spotlight. This perspective covers current knowledge of the role of neovascularization during GVHD as well as GVT and its implications for HSCT.
Vasculature during GVHD
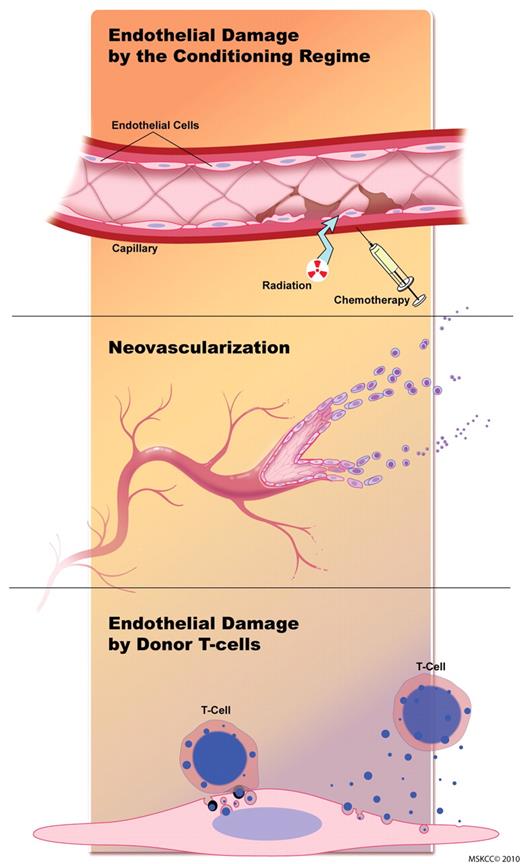
GVHD is a potentially lethal complication in patients undergoing allogeneic HSCT. It is characterized by damage of predominantly epithelial tissues in target organs caused by allo-activated T cells recognizing host tissue antigens. However, vascular pathologic processes, such as neovascularization and endothelial damage, play important roles during GVHD. The vasculature is sequentially affected during GVHD (Figure 1). Endothelial damage is caused (1) initially by the conditioning regimen, (2) in the second phase neovascularization and recruitment of inflammatory cells occur, and (3) in the third phase alloreactive T cells target the endothelium and blood vessels are destroyed. Studies on neovascularization during GVHD are summarized in Table 1.
The endothelium is sequentially affected during GVHD. Initial endothelial damage is caused by the conditioning regimen. During the second phase, neovascularization and recruitment of inflammatory cells occur. During the later stages of GVHD, alloreactive T cells target the endothelium, and blood vessels are destroyed. Figure by Terry Helms from Medical Graphics at Memorial Sloan-Kettering Cancer Center.
The endothelium is sequentially affected during GVHD. Initial endothelial damage is caused by the conditioning regimen. During the second phase, neovascularization and recruitment of inflammatory cells occur. During the later stages of GVHD, alloreactive T cells target the endothelium, and blood vessels are destroyed. Figure by Terry Helms from Medical Graphics at Memorial Sloan-Kettering Cancer Center.
Initial endothelial damage by the conditioning regimen
Radiation and chemotherapy are used as conditioning regimens, and both cause endothelial damage in many organs, including the lung, the intestines and the brain.1,2 In murine models, chemotherapy regimens that are often used in clinical HSCT, for example, cyclophosphamide (60 mg/kg, days 1 and 2) or methotrexate (15 mg/m2, days 1, 3, 6, and 11), were found to increase the number of circulating endothelial cells (ECs), which are measured to estimate endothelial damage.3 Both cyclophosphamide and methotrexate cause significant apomorphosis, hydropsia, and cytomembrane damage in ECs.3 Radiation activates ECs in vitro and in vivo in doses that are clinically applied as HSCT conditioning (2-12 Gy).4,5 Radiation with 7.5 Gy, which is lower than the standard “full-dose” conditioning with 12-Gy total body irradiation, was found to induce persistent anatomic changes in the endothelium, including intracellular edema and occlusion of microvascular lumens by edematous ECs.6 Human studies show that the intensity of the conditioning regimen positively correlates with endothelial damage, as assessed by plasma levels of VWF, a disintegrin and metalloprotease with thrombospondin domain 13 activity, soluble VCAM-1, and soluble TNF receptor I.7 The level of cyclic GMP, which is also an indicator for severe endothelial damage, was found to be increased after total body irradiation in a subset of patients undergoing HSCT. An elevated cyclic GMP level was a negative predictive factor for survival after HSCT, suggesting that endothelial damage plays a significant role in posttransplantation morbidity and mortality.8 Calcineurin inhibitors, in particular cyclosporine A (CSA), may further aggravate endothelial damage caused by the conditioning regimen.9 Taken together these findings show that the conditioning regimen (irradiation or chemotherapy or both) as well as CSA may damage host ECs. The early endothelial damage probably contributes to the initiation of processes that lead to neovascularization and inflammation that characterize GVHD.
Neovascularization during GVHD
The new formation of blood vessels in adults is termed neovascularization. Neovascularization is either mediated by angiogenesis, the proliferation of resident tissue ECs, or vasculogenesis, the incorporation of vascular endothelial progenitor cells (EPCs). It was discovered in the early 1970s that angiogenesis by capillary sprouting of host vessels is important for growth of malignant tumors.10 During capillary sprouting, vessels dilate and become leaky in response to several factors, including vascular permeability factor and vascular endothelial growth factor (VEGF).11 Angiopoetin-2 is involved in the detachment of pericytes and loosening of the matrix. Various factors stimulate endothelial proliferation during angiogenesis, including VEGF, fibroblast growth factor, TGF-β1, TNF-α, platelet-derived growth factor, and several chemokines.11 The importance of angiogenesis for malignant diseases and for a variety of inflammatory diseases is underlined by the clinical efficacy of substances that inhibit angiogenesis to treat cancer and inflammation.12-15 Vasculogenesis by BM-derived EPCs plays a role during embryogenesis, and recent data suggest that it is also important for tumor vasculature in adults, although there is controversy about this issue.16-18 EPCs are a subset of BM resident cells, which are probably derived from HSCs and express progenitor markers as well as endothelial antigens. Mobilization of EPCs from the BM to the peripheral blood is regulated by various factors and can occur during inflammation, tumor growth, ischemia, and vascular trauma.19 EPCs preferentially home to activated endothelium with a high level of adhesion molecule expression. After homing to the endothelium, EPCs are inserted into the monolayer of surrounding mature vascular ECs, which may lead to the formation of new blood vessels.
It was discovered early that graft-versus-host (GVH) reactions are associated with increased neovascularization. In the late 1960s and early 1970s Brent et al,20,21 Streilein and Billingham,22 and Zakarian and Billingham23 injected allogeneic lymphocytes and found local GVH reactions characterized by local swelling, induration of the skin, and erythema. In the mid 1970s Sidky and Auerbach24 analyzed in detail the host local vascular response after irradiation and intracutaneous allogeneic lymphocyte transfer. This work was performed in the context of a lack of reliable methods to predict and measure the strength of GVH reactions. The investigators hypothesized that the assessment of the host local vascular response can serve as a quantitative measure of GVH reactions. They irradiated HalCr mice (8 Gy) and intracutaneously injected allogeneic BALB/c splenocytes or syngeneic splenocytes. With the use of a dissecting microscope the vascular density was assessed by counting the number of vessels per field. When immunocompetent cells were injected into histoincompatible hosts, the scar region became surrounded by a network of blood vessels as early as 48 hours after injection. Interestingly, the number of allogeneic splenocytes that were injected correlated directly with the amount of neovascularization. Intracutaneous injection of syngeneic splenocytes did not result in neovascularization.
Despite these early studies on vascular proliferation during local GVH reactions, the role of neovascularization during GVHD has not been studied experimentally until very recently. We used murine GVHD models to assess neovascularization,25 following the hypothesis that neovascularization plays an important role during GVHD and can be used as a therapeutic target. In lethally irradiated HSC transplant recipients we found higher vessel density in the liver, ileum, and colon during GVHD. With the use of flow cytometry we found that neovascularization was because of donor-derived ECs. We next adoptively transferred selected green fluorescence protein–positive EPCs and observed incorporation into the neovasculature of the inflamed intestines and liver during GVHD. Taken together, these data show that GVHD is characterized by neovascularization, which is mainly driven by vasculogenesis as opposed to angiogenesis. The predominant role that donor ECs play in the formation of neovasculature after HSCT in our models is not surprising because of the negative effect that lethal doses of irradiation have on host EC function.26-33 It has been shown that irradiation doses similar to those used clinically are sufficient to inhibit EC function in allo-BM transplant recipients.6 Future experimental studies should determine the role of vasculogenesis versus angiogenesis during GVHD in HSCT models with the use of chemotherapy as opposed to lethal irradiation.
Results from human studies are in line with the findings in murine models showing the presence of neovascularization during GVHD. Several studies have shown that GVHD is associated with neovascularization in target organs, such as the intestines and the skin. The vascular density in samples from gastric biopsies was found to be greater in patients with GVHD than in samples from patients with gastritis and in healthy controls.34 In the histopathologic analysis of skin biopsies, signs of vascular proliferation were significantly more common in acute cutaneous GVHD than in control skin biopsies from HSC transplant recipients free of GVHD.35
Regarding the importance of vasculogenesis versus angiogenesis in human GVHD a number of studies support the hypothesis that vasculogenesis contributes to neovasculature in GVHD target organs. Lin et al36 investigated circulating ECs in HSC transplant recipients. In peripheral blood, they found host-derived ECs as well as donor-derived ECs. However, only the donor-derived circulating ECs had a high capacity to proliferate in cultures. These findings suggest that circulating donor-derived ECs and their progenitors, as opposed to host-derived ECs, contribute to blood vessel growth after HSCT.
In line with these results, there is a series of clinical studies that showed that donor BM-derived vasculogenesis contributes to neovascularization in the skin and intestines during GVHD.37-39 These studies used a combination of XY FISH and immunostaining in skin or gut biopsies from sex-mismatched female transplant recipients (with male donors). In skin biopsy samples ECs of donor origin were considerably increased in patients with GVHD.39 Our group also studied the donor-versus-recipient origin of ECs in the skin of sex-mismatched HSC transplant recipients.38 Combining FISH 3-dimensional tissue Z-stack analysis of double immunostaining, we found ECs of donor origin, but only in patients with GVHD in areas of severe GVHD tissue damage.
There are 2 possible mechanisms how BM-derived EPCs may directly contribute to vasculogenesis after HSCT: (1) fusion between donor EPCs and host ECs or (2) differentiation of donor EPCs to ECs. To clarify the mechanism, Jiang et al37 performed XY FISH and immunohistochemistry in gut and skin biopsies of sex-mismatched transplant recipients. Donor-derived ECs were detected in the skin and gut of transplant recipients with a mean frequency of 2%. None of the > 4000 ECs examined had > 2 sex chromosomes, consistent with an absence of cell fusion. This finding is in line with our own data (O.P. and M.v.d.B., unpublished data, November 2008): In a MHC-mismatched murine HSCT model (donor H2kB and recipient H2kD) we specifically looked for cell fusion events that would lead to coexpression of the MHC molecules H2kB and H2kD on ECs detectable with the use of flow cytometry. ECs in GVHD target organs were always single positive (either H2kB or H2kD) for the donor-host markers. Therefore, differentiation of EPCs to ECs, rather than cell fusion, appears to be the main mechanism of vasculogenesis during GVHD.
In conclusion, the human studies support the experimental data showing that acute GVHD is associated with increased neovascularization (Table 1). Experimental data suggest that neovascularization in GVHD target organs after HSCT is mediated primarily by donor-derived vasculogenesis as opposed to host-derived angiogenesis. Clinical studies confirm that donor-derived vasculogenesis contributes to neovascularization during GVHD. However, clinical studies did not permit any quantitative assessment of the relative contributions of vasculogenesis versus angiogenesis.
Studies on neovascularization during GVHD
| Model/observation . | References . |
|---|---|
| Animal | |
| Local GVH reactions occur after injection of allogeneic lymphocytes. | 20,,–23 |
| When immunocompetent splenocytes are injected intracutaneously into histoincompatible hosts, neovascularization occurs. There is a positive correlation between the number of injected cells and the degree of neovascularization. | 24 |
| GVHD is associated with neovascularization in target organs. | 25 |
| In irradiated hosts with GVHD neovascularization is due to vasculogenesis that is mediated by donor-derived endothelial progenitor cells. | 25 |
| The inhibition of neovascularization with monoclonal antibodies against monomers of vascular endothelial cadherin ameliorates GVHD. | 25 |
| Anti-VEGFR1/anti-VEGFR2 antibodies after allo-BM transplantation inhibit hematopoietic reconstitution. | 25 |
| Human | |
| Circulating endothelial cells are increased in HSC transplant recipients. Only donor-derived circulating endothelial cells have a high capacity to proliferate. | 36 |
| Donor BM-derived vasculogenesis contributes to neovascularization in the skin during GVHD. | 37,–39 |
| Donor BM-derived vasculogenesis contributes to neovascularization in the intestines during GVHD. | 37 |
| Donor-derived endothelial cells are more numerous and preferentially distributed in the areas of severe acute GVHD damage. | 38 |
| In gastric biopsies the vascular density is greater in patients with acute GVHD than in healthy controls. | 34 |
| In skin biopsies from patients with acute GVHD, there are areas with high vascular density. | 35 |
| There is a positive correlation between a low VEGF serum level and the occurrence of GVHD. | 40 |
| Single nucleotide polymorphisms leading to low VEGF production are associated with a higher incidence of GVHD. | 41 |
| High VEGF serum levels after HSCT are associated with less severe GVHD (there was a trend; however, the association was statistically not significant). | 42 |
| Treatment with bevacizumab (anti–VEGF-A mAb) before autologous HSCT has no major negative effect on hematopoietic reconstitution. | 43 |
| Model/observation . | References . |
|---|---|
| Animal | |
| Local GVH reactions occur after injection of allogeneic lymphocytes. | 20,,–23 |
| When immunocompetent splenocytes are injected intracutaneously into histoincompatible hosts, neovascularization occurs. There is a positive correlation between the number of injected cells and the degree of neovascularization. | 24 |
| GVHD is associated with neovascularization in target organs. | 25 |
| In irradiated hosts with GVHD neovascularization is due to vasculogenesis that is mediated by donor-derived endothelial progenitor cells. | 25 |
| The inhibition of neovascularization with monoclonal antibodies against monomers of vascular endothelial cadherin ameliorates GVHD. | 25 |
| Anti-VEGFR1/anti-VEGFR2 antibodies after allo-BM transplantation inhibit hematopoietic reconstitution. | 25 |
| Human | |
| Circulating endothelial cells are increased in HSC transplant recipients. Only donor-derived circulating endothelial cells have a high capacity to proliferate. | 36 |
| Donor BM-derived vasculogenesis contributes to neovascularization in the skin during GVHD. | 37,–39 |
| Donor BM-derived vasculogenesis contributes to neovascularization in the intestines during GVHD. | 37 |
| Donor-derived endothelial cells are more numerous and preferentially distributed in the areas of severe acute GVHD damage. | 38 |
| In gastric biopsies the vascular density is greater in patients with acute GVHD than in healthy controls. | 34 |
| In skin biopsies from patients with acute GVHD, there are areas with high vascular density. | 35 |
| There is a positive correlation between a low VEGF serum level and the occurrence of GVHD. | 40 |
| Single nucleotide polymorphisms leading to low VEGF production are associated with a higher incidence of GVHD. | 41 |
| High VEGF serum levels after HSCT are associated with less severe GVHD (there was a trend; however, the association was statistically not significant). | 42 |
| Treatment with bevacizumab (anti–VEGF-A mAb) before autologous HSCT has no major negative effect on hematopoietic reconstitution. | 43 |
These findings in experimental models as well as in humans may be clinically significant because of their potential implications for therapies targeting neovasculature after HSCT. To date most antineovascularization therapies, which are used clinically or preclinically against cancer or inflammatory diseases, inhibit angiogenesis. The potential effect of drugs targeting VEGF, such as bevacizumab, on vasculogenesis warrants further experimental data and clinical studies. One could hypothesize that anti-VEGF treatment inhibits vasculogenesis because VEGF is highly expressed on circulating EPCs; however, this has not been studied experimentally. The predominant role of vasculogenesis in the formation of neovasculature during GVHD makes it a suitable target for selective therapies. Because many physiologic processes, for example, wound healing and tissue regeneration, depend on angiogenesis, it is reasonable to believe that the specific inhibition of vasculogenesis has fewer unwanted effects compared with the inhibition of angiogenesis. The perception that neovascularization plays a role in GVHD pathophysiology prompted several studies that investigated VEGF levels and VEGF single nucleotide polymorphisms. A positive correlation between a low VEGF level and the occurrence of GVHD was found in patients undergoing HSCT.40 Another study correlated single nucleotide polymorphisms leading to a lower VEGF production with a higher incidence of GVHD.41 In line with these results it was shown that high VEGF levels after HSCT were associated with a trend toward less-severe acute GVHD.42 These clinical results suggest a correlation between low VEGF production and the severity of GVHD in HSC transplant recipients. However, the mechanism of this connection is unclear, and the clinical results are currently not supported by experimental data. In murine HSCT models, we found that VEGF genes were neither up-regulated nor down-regulated during GVHD.25 Further experimental studies in animal models are needed to clarify the mechanism of the correlation between VEGF production and GVHD in patients undergoing HSCT. In future experiments it will be particularly important to investigate the effect of monoclonal Abs (mAbs) that target murine VEGF (eg, G6-31) and to use VEGF-deficient mice as allo-HSCT donors or recipients or both.
Vasculogenesis does also play a role in in solid-organ transplantation; several investigators have demonstrated that BM-derived EPCs participate in the formation of neovasculature in allografts.44 After human cardiac transplantation45 and after human renal transplantation46 as many as 20% of donor vascular ECs were found in the allograft. The percentage of BM-derived ECs was highest after acute vascular allograft rejection.46
Neovascularization as therapeutic target in GVHD
The inhibition of neovascularization has been successfully used therapeutically in inflammatory diseases, such as inflammatory bowel disease, arthritis, and dermatitis.12-14 As mentioned earlier, vasculogenesis, as opposed to angiogenesis, plays a predominant role in the formation of neovasculature during GVHD after lethal irradiation. However, whether donor ECs simply reflect a wound-healing process or an active pathologic process is currently unknown. Only in the latter case it would be logical to use inhibitors of neovascularization as a GVHD therapy.
We hypothesized that the inhibition of neovascularization could prevent the development of GVHD, suggesting that neovascularization during GVHD is an active pathologic process. To specifically inhibit vasculogenesis, we used an antibody (E4G10), which recognizes vascular endothelial cadherin monomers on EPCs.25 We observed that administration of E4G10 was associated with a significant inhibition of donor BM-derived neovascularization in the liver, ileum, and colon during GVHD. E4G10-treated HSC transplant recipients had better survival, less target organ damage, reduced numbers of tissue-infiltrating CD3+ T cells, and lower clinical GVHD scores in different murine GVHD models. The main mechanism of the therapeutic efficacy of the inhibition of neovascularization to reduce inflammation is probably the impaired recruitment of proinflammatory cells migrating via the blood vessels to inflammatory sites. However, ECs have many in vivo functions, and further evidence from animal studies during GVHD with particular focus on the role of different cell types during vasculogenesis, such as EPCs versus myeloid cells, are needed to gain knowledge about the mechanisms of the interplay between neovascularization and inflammation. In animal models there are several established methods to genetically or pharmacologically deplete circulating EPCs, including the use of Inhibitor of DNA binding 1 (ID-deficient mice),17 ID antagonism47 and the use of antibodies against vascular endothelial cadherin monomers.25,48 Furthermore, it will be important to investigate the effects of the adoptive transfer of selected donor EPCs after allo-HSCT on the development of GVHD. Aggravation of GVHD as a result of EPC transfer would support the hypothesis that EPCs are mediators of vasculogenesis during GVHD. The investigation of the specific contribution of myeloid cells to vasculogenesis in GVHD animal models might prove to be more difficult because a global depletion of myeloid cells during GVHD, for example, liposomal clodronate has multiple negative effects, including a higher susceptibility to infections, and may lead to shorter survival.49,50 There are, however, several substances/pathways that could be useful to specifically target the migration or function of myeloid cells in preclinical GVHD models. (1) Prokineticin-2 (Bv8) is an important proangiogenic factor that is produced by myeloid cells. Monoclonal antibodies against Bv8 lead to impaired recruitment of myeloid cells to tumor neovasculature and to inhibition of neovascularization.51 (2) Matrix metalloproteinase 9 is another proangiogenic factor produced by myeloid cells. Genetic or pharmacologic antagonism of matrix metalloproteinase 9 leads to impaired neovascularization during tumor growth52 and inflammatory diseases, such as bronchial asthma53 and inflammatory bowel disease.54,55 (3) The tyrosine kinase receptor CSF receptor-1 (CSF-1R, CD115) regulates the recruitment of myeloid cells to tumors as well as to inflammation sites and can be used as a therapeutic target to inhibit myeloid cell–mediated vasculogenesis.56
To test whether VEGF could be used as a therapeutic target during GVHD, we used anti-VEGFR1/anti-VEGFR2 antibodies after allo-BMT and found an inhibitory effect on hematopoietic reconstitution, leading to early death of allo-BM transplant recipients.25 Furthermore, we found that VEGF was not overexpressed during GVHD in target tissues. These results suggest that the use of anti-VEGF strategies for prevention of GVHD may not be effective and may potentially inhibit hematopoietic reconstitution. However, one recently published small clinical study used bevacizumab (anti–VEGF-A mAb) in patients with sarcoma undergoing autologous HSCT without apparent negative effect on reconstitution.43 Sixteen patients received 7.5 or 10 mg/kg bevacizumab at day −5 of HSCT in combination with ifosfamide, carboplatin, and etoposide, and no delay of hematopoietic reconstitution was seen. The use of bevacizumab before HSCT, as opposed to using bevacizumab after HSCT, could explain the discrepancies in the inhibition of reconstitution between the preclinical models and the clinical study.
Future studies should investigate neovascularization not only in GVHD target organs but also in lymphoid organs because the inhibition of lymphatic vessel growth may affect the activation and proliferation of immune cells, such as alloreactive T cells, during GVHD. A recent report shows that the specific inhibition of lymphangiogenesis with a mAb against VEGFR3 increases the severity of inflammation in a mouse model of chronic inflammatory arthritis.57 In line with these results, another study found that activation of the VEGFR3 pathway by VEGF-C attenuates skin inflammation by promoting lymphangiogenesis.58 However, treatment with VEGFR3 mAb reduced the level of tissue-infiltrating alloreactive T cells in a cardiac allograft model, suggesting that inhibition of lymphangiogenesis may lead to reduced inflammatory reactions in the setting of histoincompatibility.59 Taken together, it is currently hard to predict if lymphangiogenesis could be a therapeutic target during GVHD.
Another approach to inhibit GVHD with substances that inhibit neovascularization is the administration of proteasome inhibitors. Bortezomib has been shown to be a potent inhibitor of angiogenesis.60 Two different groups reported that the early administration of bortezomib, a proteasome inhibitor, protects against the development of acute GVHD in murine HSCT models.61,62 These reports are in line with a promising clinical report that investigated bortezomib as GVHD prophylaxis in patients undergoing HSCT.63 However, proteasome inhibitors have multiple in vivo effects, in particular the proteasome has been shown to play a role in T-cell activation, proliferation, and apoptosis.64 The above-mentioned studies on bortezomib in experimental GVHD have not assessed neovascularization as a possible effect of bortezomib efficacy. Therefore, it is not clear if the inhibition of neovascularization by bortezomib is relevant to its positive effects on GVHD. Further studies on bortezomib in GVHD mouse models, specifically designed to investigate neovascularization, are needed to clarify if the activity of bortezomib is mainly, or in part, based on the inhibition of neovascularization.
Currently, there are no clinical studies available to investigate the efficacy of drugs specifically targeting neovasculature in the prevention or treatment of GVHD. However, several established drugs for GVHD prophylaxis, such as CSA, methotrexate, and mycophenolate mofetil (MMF), inhibit neovascularization besides having multiple other effects in vivo. In murine models of corneal neovascularization CSA inhibited the migration of primary ECs and reduced angiogenesis induced by VEGF.65 Furthermore, CSA inhibits EC function in vitro66 and was found to cause endothelial dysfunction in animal studies that used capillary tube assays.67 Methotrexate also inhibited EC proliferation in vivo in a model for corneal neovascularization.68 Several groups have found that MMF reduces EC proliferation and neovascularization in vitro as well as in vivo.69,70 These data suggest that the inhibition of neovascularization might contribute to the inhibitory activity of CSA, methotrexate, and MMF in the development of GVHD.
Endothelial damage during GVHD
Endothelial damage is a pathologic hallmark of vascular complications after HSCT, such as veno-occlusive disease of the liver, thrombotic microangiopathy, and capillary leak syndrome. Although acute GVHD is classically considered to be an “epithelial” disease, both the presence of cutaneous erythema and gastrointestinal bleeding led to the hypothesis that the vasculature may be directly or indirectly damaged during GVHD.35 Disseminated EC apoptosis was the first detectable lesion in a murine model of acute tissue damage induced by systemic transfer of allogeneic lymphocytes, suggesting that vascular lesions play an important role in the pathogenesis of allogeneic immune responses.71 In another murine model of acute GVHD that did not involve any conditioning treatment, the earliest detectable oral mucosa lesion was apoptosis of the ECs from chorionic capillaries, which precedes basal keratinocyte apoptosis.72 Moreover, EC death and lymphocytic inflammation preceded epithelial injury during the development of acute GVHD. These findings collectively show that ECs are damaged by activated alloreactive donor T cells. During GVHD host hematopoietic antigen presenting cells play an important role in the activation of donor T cells.73 Alloantigen presentation by hematopoietic professional APCs is, however, not required for activation of allogeneic T cells. Vascularized cardiac allografts are acutely rejected via CD8+ direct allorecognition even if the alloantigen is not presented by hematopoietic APCs.74 This can happen because ECs are able to present antigens to T cells potently through different pathways. Through a direct pathway and through an indirect pathway liver sinusoidal ECs are capable of cross-presenting soluble exogenous antigen to CD8+ T cells.75 Of note, ECs do not always effectively activate alloreactive T cells. There is the possibility that antigen in the vasculature can be immunologically ignored. Lakkis et al76 found in cardiac allograft models that alloimmune responses to a vascularized organ transplantation were not initiated in the graft itself. In recipients lacking secondary lymphoid organs they demonstrated that the permanent acceptance of allografts was because of immunologic “ignorance.” Another group found in a GVHD-like model and in solid-organ transplantation models that CD8+ T-cell responses against minor antigens were not initiated by ECs in the absence of dendritic cells.77
In a human study of intestinal GVHD, pericapillary hemorrhage was shown in areas with EC lesions, and this severe form of intestinal GVHD was associated with severe hemorrhagic enterocolitis.78 The endothelial damage during GVHD may be intensified by prophylaxis with calcineurin inhibitors that cause injury to ECs.79
In later stages of GVHD, the destruction of vasculature leads to rarefaction of blood vessels in target organs. In murine models of GVHD, we found that the vascular density in the intestines decreased day 30 after HSCT (O.P and M.v.d.B., unpublished observation, November 2008). This finding is in line with a report on patients with sclerotic chronic GVHD of the skin.80 Here, cutaneous microvessel loss was identified as a hallmark feature and was associated with an infiltration of CD8 T lymphocytes into the upper dermis.80 In line with these results it was shown that during chronic GVHD the number of circulating EPCs is decreased.81 Patients with chronic sclerodermatous GVHD also had impaired endothelial-forming ability compared with patients after HSCT without chronic sclerodermatous GVHD. However, the rarefaction of vessels in the skin during chronic GVHD seems to be less pronounced compared with systemic sclerosis.82 Areas of microvascular endothelial proliferation were present in the biopsies taken at relatively early times during chronic GVHD but not in late biopsies.82
Because neovascularization and endothelial damage occur during GVHD, markers of endothelial biology may be helpful in the diagnosis of GVHD. Circulating ECs, endothelial microparticles, and EC markers, are rarely found in the peripheral blood of healthy persons and increase when endothelial injury occurs.83 In patients after myeloablative HSCT with busulfan/cyclophosphamide, circulating ECs were found to continuously increase until day 21.84 Pihusch et al85 analyzed EC-derived microparticles in HSC transplant recipients. Microparticles were not significantly influenced by the conditioning regimen or by infectious complications. However, in patients with GVHD significantly higher levels of microparticles were detected compared with the controls after HSCT without GVHD. Endothelial microparticles may contribute to inflammation because they induce maturation and activation of plasmacytoid dendritic cells.86 The same group also showed that the EC markers VWF and thrombomodulin are elevated in the peripheral blood of patients with GVHD that received an HSC transplant.87 These results suggest that markers of EC biology might serve as a diagnostic test for differentiation of GVHD from other transplantation-related complications. This underlines the importance of vascular processes for the pathophysiology of GVHD.
Activation, targeting, and damage of ECs are not only critical for GVHD, but these processes are also important mechanisms during allograft rejection in solid-organ transplantation. Allograft rejection involves recruitment and activation of circulating leukocytes in response to activated microvascular ECs.88 During rejection class I MHC molecules on graft ECs are recognized by host alloactivated T cells, leading to endothelial damage. The replacement of graft endothelium by recipient BM-derived ECs was found to be required for allograft rejection by CD4+ T cells, underlining the significance of vasculogenesis for alloimmunity.89
Vasculature and graft-versus-tumor activity
It is well accepted that the inhibition of angiogenesis as well as of vasculogenesis can inhibit tumor growth, and several inhibitors of angiogenesis are already in clinical use as cancer therapies.15 More recently, knowledge has been gained about the inhibition of neovascularization as a mechanism of action of T-cell therapies against cancer. There is an increasing body of evidence showing that T cells not only directly interact with tumor cells but also target tumor vasculature during allogeneic immune responses against malignancies.
To investigate the role of neovascularization in malignancies in HSC transplant recipients we used murine HSCT models with acute myeloid leukemia, B lymphoma, and renal carcinoma.25 We found that neovascularization mediated by donor-derived EPCs played a significant role in tumor growth after HSCT and lethal radiation. In HSCT models without graft-versus-tumor (GVT) activity (without donor T cells) we found a moderate inhibitory effect of the pharmacologic inhibition of vasculogenesis on tumor growth. We found inhibition of tumor growth in a solid-tumor model (renal carcinoma) as well as in hematologic malignancies (lymphoma and acute myeloid leukemia). Our results are in agreement with recent clinical data, suggesting a role for neovascularization not only in solid tumors but also in hematologic malignancies.90 In patients after HSCT, relapse is more likely to involve vasculogenesis than angiogenesis, because angiogenesis is compromised because of damage to the vasculature from conditioning. This situation could be similar to malignant glioma in which primary tumors induce angiogenesis, whereas relapse after radiation therapy induces vasculogenesis.91
In contrast to the rather moderate effects on tumors in HSCT models without GVT, we found a stronger therapeutic effect of the inhibition of neovascularization on survival in HSCT models with GVT. We are currently performing studies to find out the main mechanism of the enhancement of GVT effects by inhibitors of neovascularization. One possible explanation for the enhancement of GVT activity through inhibitors of neovascularization is a normalization of tumor vasculature that increases the blood flow and leads to a more effective recruitment of tumor-reactive T cells to the tumor tissue.92,93 Another explanation is the enhancement of leukocyte infiltration in tumors after antiangiogenic therapy. Several inhibitors of neovascularization including anginex, endostatin, and angiostatin, were found to stimulate leukocyte–vessel wall interactions and to increase leukocyte infiltration in tumor tissues.94 These results suggest that immunotherapy strategies, including HSCT, may be improved by combination with reagents that inhibit neovascularization.
As mentioned earlier, several studies have shown that the inhibition of neovascularization contributes to the antitumor effects of T-cell therapies. In animal models that use syngeneic and allogeneic tumors it was shown that tumor rejection depends on stromal events affecting the tumor environment.95 The damage of tumor neovasculature, mediated by host leukocytes, was a prerequisite to tumor rejection. Qin and Blankenstein96 showed that CD4+ immunity against an MHCII-tumor depends on the inhibition of tumor angiogenesis as a result of IFNγ release. They used different primary tumors from IFNγ-R+/− as well as from IFNγ-R−/− mice. For tumor rejection IFNγR expression was necessary only in the effector phase on nonhematopoietic cells. In IFNγR−/− mice, tumor blood vessels were observed at early time points. In contrast, in IFNγR+/− mice, blood vessels within the tumor mass were completely absent, and the tumor mass became necrotic. The investigators concluded that CD4+ T cell–dependent tumor immunity involves tumor destruction indirectly by the inhibition of angiogenesis. Another group has shown that T Ag–specific CD4+ T cells homed selectively into the tumor microenvironment in an animal model for pancreatic carcinoma.97 CD4+ T cells inhibited tumor neovascularization through release of antiangiogenic chemokines. Combined TNFR1 and IFN-γ signaling was found to be involved in the antiangiogenic activity. Results of another study are clinically relevant to GVT reactions in patients undergoing HLA-matched HSCT98 ; transferred CD8+ T cells primed against a minor antigen (H7a) lead to tumor rejection in melanoma-bearing mice. Tumor rejection was initiated by preferential extravasation at the tumor site of IFNγ-producing H7a-specific T cells, leading to inhibition of tumor neovascularization.
Taken together, these studies support the hypothesis that inhibition of neovascularization contributes to the beneficial GVT activity after HSCT.
Conclusions and future directions
Results from animal models as well as clinical data show that neovascularization is involved in GVHD, tumor growth, and GVT activity after HSCT. The initiation phase of GVHD is characterized by increased neovascularization and by recruitment of inflammatory cells. In the course of the disease the vasculature is targeted by alloreactive donor T cells, and vascular destruction occurs. Tumor growth after HSCT depends on neovascularization, and the inhibition of neovascularization contributes to the GVT activity of HSCT. Alloreactive donor T cells infiltrate the tumor stroma and secrete antiangiogenic substances, consequently leading to inhibition of tumor neovascularization and tumor cell death.
The therapeutic concept of the inhibition of neovascularization is promising because of its simultaneous beneficial effects on GVHD and GVT. A key feature after HSCT is that vasculogenesis by donor BM-derived cells contributes to neovascularization, which influences therapeutic concepts to inhibit neovascularization. First results of animal studies have shown that amelioration of GVHD and inhibition of tumor growth is achievable by therapeutic targeting of neovascularization, in particular by targeting vasculogenesis. However, the optimal compounds as well as the best times to inhibit neovascularization after HSCT have not been determined, and clinical studies are not yet available. Approaches targeting neovascularization in HSC transplant recipients could provide novel strategies to prevent or treat GVHD and to decrease relapse after transplantation.
Authorship
Contribution: O.P., G.S., and M.R.M.v.d.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olaf Penack, Charité University Hospital, Department of Hematology and Oncology, Hindenburgdamm 30, 12200 Berlin, Germany; e-mail: olaf.penack@charite.de.