Abstract
G6PC3 (or glucose-6-phosphatase-β) deficiency underlies a congenital neutropenia syndrome in which neutrophils exhibit enhanced endoplasmic reticulum (ER) stress, increased apoptosis, impaired energy homeostasis, and impaired functionality. Here we show that murine G6pc3−/− neutrophils undergoing ER stress activate protein kinase-like ER kinase and phosphatidylinositol 3,4,5-trisphosphate/Akt signaling pathways, and that neutrophil apoptosis is mediated in part by the intrinsic mitochondrial pathway. In G6PC3-deficient patients, granulocyte colony-stimulating factor (G-CSF) improves neutropenia, but its impact on neutrophil apoptosis and dysfunction is unknown. We now show that G-CSF delays neutrophil apoptosis in vitro by modulating apoptotic mediators. However, G6pc3−/− neutrophils in culture exhibit accelerated apoptosis compared with wild-type neutrophils both in the presence or absence of G-CSF. Limiting glucose (0.6mM) accelerates apoptosis but is more pronounced for wild-type neutrophils, leading to similar survival profiles for both neutrophil populations. In vivo G-CSF therapy completely corrects neutropenia and normalizes levels of p-Akt, phosphatidylinositol 3,4,5-trisphosphate, and active caspase-3. Neutrophils from in vivo G-CSF–treated G6pc3−/− mice exhibit increased glucose uptake and elevated intracellular levels of G6P, lactate, and adenosine-5′-triphosphate, leading to improved functionality. Together, the results strongly suggest that G-CSF improves G6pc3−/− neutrophil survival by modulating apoptotic mediators and rectifies function by enhancing energy homeostasis.
Introduction
Glucose-6-phosphatase-β (G6Pase-β, also known as G6PC3) is a ubiquitously expressed glucose-6-phosphate (G6P) hydrolase that catalyzes the hydrolysis of G6P to glucose.1 The enzyme is an endoplasmic reticulum (ER) transmembrane protein, with its active site lying inside the ER lumen.2 G6Pase-β activity depends on the translocation of G6P from the cytoplasm into the lumen of the ER by the G6P transporter (G6PT).3-5 Deficiencies in G6PT cause glycogen storage disease type Ib (GSD-Ib), characterized by impaired glucose homeostasis, neutropenia, and neutrophil dysfunction.4,5 Although the loss of glucose homeostasis in GSD-Ib is well understood,4,5 the basis for neutropenia and neutrophil dysfunction was not clear. Kuijpers et al6 showed that neutrophils from GSD-Ib patients exhibit enhanced apoptosis, but the underlying mechanism remained unclear. Recently, we demonstrated that neutrophils express both G6Pase-β and G6PT,7 and hypothesized that, if endogenous glucose production in the ER via the G6Pase-β/G6PT complex is critical for neutrophil homeostasis and function, a detrimental mutation in either G6Pase-β or G6PT could prevent the other functioning efficiently, leading to the common myeloid phenotype. Three recent studies provide experimental support for this hypothesis. One showed that G6Pase-β–deficient (G6pc3−/−) mice manifest neutropenia and neutrophil dysfunction. Their neutrophils exhibit impaired respiratory burst, chemotaxis, and calcium flux, mimicking the immune phenotype seen in human GSD-Ib patients.7 The study also showed that G6pc3−/− neutrophils exhibited enhanced ER stress and apoptosis that underlie, at least in part, neutropenia in murine G6PC3 deficiency.7 This finding correlated with a report that a severe human congenital neutropenia syndrome, characterized by enhanced neutrophil ER stress and apoptosis, contained mutations in the human G6PC3 gene.8 A third study showed that neutrophils from GSD-Ib mice exhibit enhanced ER stress and apoptosis.9
The fate of cells under ER stress is dependent on the balance between cell adaptive and cell death responses.10,11 In response to ER stress, the protein kinase-like ER kinase (PERK) signaling pathway is activated10,11 along with the phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3]/Akt cell survival signaling pathway.12,13 Prolonged ER stress leads to apoptotic cell death,10,11 and the intrinsic apoptosis pathway, programmed for a rapid spontaneous cell death, appears to be the dominant pathway in neutrophils.14 We have previously shown that neutrophil apoptosis in GSD-Ib mice is mediated by the intrinsic mitochondrial stress pathway.9 In this study, we investigate the signaling pathways of ER stress and apoptosis in G6pc3−/− neutrophils.
Granulocyte colony-stimulating factor (G-CSF) is widely used to treat neutropenia in a variety of conditions, including GSD-Ib15,16 and G6PC3 deficiency.8,17 G-CSF therapy improves neutrophil counts in both disorders and decreases the number and severity of bacterial infections. This cytokine is also known to delay neutrophil apoptosis by modulating apoptotic mediators.18-20 G-CSF increases the expression of the antiapoptotic protein Mcl-1,18 blocks Bax translocation to the mitochondria,19 and prevents the activation of caspase-9 and caspase-3.19,20 Surprisingly, G-CSF fails to delay apoptosis of human GSD-Ib neutrophils when cultured in vitro.6 The functional dependence between G6PT and G6Pase-β, and the near-identical myeloid phenotype in GSD-Ib4,5,9 and G6PC3 deficiency,7,8,17,21 prompted us to examine the impact of G-CSF on apoptosis of in vitro cultured G6pc3−/− neutrophils.
Both G6PC3-deficient7,17,21 and GSD-Ib4,5 neutrophils exhibited dysfunction characterized by impaired respiratory burst, chemotaxis, and calcium flux activities. In vivo G-CSF therapy improved neutrophil function in GSD-Ib patients,15,16,22 but the underlying mechanism is unknown. We have previously shown that G6Pase-β is essential for neutrophil energy homeostasis and neutrophils from G6pc3−/− mice, and G6PC3-deficient patients display reduced levels of intracellular G6P, lactate, and adenosine-5′-triphosphate (ATP), compared with their respective controls.21 The disruption in energy homeostasis in G6PC3-deficient neutrophils results in impaired functionality. We now hypothesize that G-CSF can stimulate glucose uptake in neutrophils, which in turn will improve neutrophil energy homeostasis and functionality.
In this study, we present evidence that: (1) PERK-mediated signaling is one pathway that mediates ER stress in G6pc3−/− neutrophils and the stress also activates the PtdIns(3,4,5)P3/Akt cell survival pathway; (2) the intrinsic mitochondrial pathway mediates apoptosis in G6pc3−/− neutrophils; (3) G-CSF significantly delays apoptosis in G6pc3−/− neutrophils by modulating apoptotic mediators; and (4) neutrophils from G-CSF–treated G6pc3−/− mice exhibit increased glucose uptake and intracellular levels of G6P, lactate, and ATP, leading to improved functionality. Together, these results provide insight into the etiology of neutropenia and neutrophil dysfunction in the congenital neutropenia syndrome, G6PC3 deficiency.
Methods
Neutrophil isolation
Animal studies were approved by the National Institute of Child Health and Human Development Animal Care and Use Committee. Bone marrow (BM) cells were isolated from the femurs and tibiae of 6- to 8-week-old congenic G6pc3−/− and control littermates in the C57BL/6J background21 using the BM Harvesting kit (Millipore). Neutrophils were purified from BM or blood using the EasySep negative selection system (StemCell Technologies).21 Freshly isolated G6pc3−/− and control BM neutrophils were of similar biochemical maturity, with both populations containing more than 95% morphologically mature neutrophils and expressing similar levels of Gr-1 and gelatinase.21 Neutrophil purity was analyzed using rat monoclonal antibodies against mouse Gr-1 conjugated with fluorescein isothiocyanate, and mouse CD11b conjugated with phycoerythrin (eBioscience). Flow cytometry was conducted with a Guava EasyCyte Mini System (Millipore) using Guava CytoSoft Version 4.2.1 (Millipore) and FlowJo, Version 7 (TreeStar). The annexin V Microbead kit (Miltenyi Biotec) was used to deplete apoptotic cells in the isolated BM neutrophils.21
Analysis of neutrophil survival, PtdIns(3,4,5)P3, p-Akt, and caspases
Annexin-V depleted BM neutrophils (4 × 106 cells/mL) were incubated in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum to yield a final glucose concentration of 0.6mM (limiting glucose). An aliquot of these cells was further adjusted to a final glucose concentration of 6.2mM (standard). Both were incubated in the absence and presence of 100 ng/mL of recombinant mouse G-CSF (Shenandoah Biotechnologies) added at 0, 12, 24, and/or 48 hours after the initiation of in vitro culturing. Separate aliquots of neutrophils in standard medium were treated with 1mM 2-deoxy-D-[1,2-3H]-glucose (2-DG; Sigma-Aldrich), 10−6M f-Met-Leu-Phe (fMLP; Sigma-Aldrich), 0.8 μg/mL recombinant mouse complement C5a (Biovision), or 5 μg/mL cytochalasin B (CytB; Sigma-Aldrich). At the indicated times, 5 × 104 cells were removed and neutrophil survival was assessed using the Guava Nexin kit (Millipore) that uses annexin V to detect early apoptotic cells and 7-aminoactinomycin (7-AAD) to detect late apoptotic or dead cells. The viable cells representing annexin V (−) and 7-AAD (−) cells were analyzed by flow cytometry.
To analyze membrane PtdIns(3,4,5)P3 expression, 105 cells were removed at the indicated times, fixed in 3.75% formaldehyde, and incubated with a mouse polyclonal antibody against PtdIns(3,4,5)P3 (Echelon Biosciences) followed with a mouse IgG antibody conjugated with AlexaFluor-488. Labeled cells were analyzed by flow cytometry. To analyze p-Akt, total PtdIns(3,4,5)P3, active caspase-3, and active caspase-9 expression, 105 cells were removed at indicated times, fixed in 3.75% formaldehyde, and permeabilized in 0.2% Triton ×-100. Cells were then incubated with the following antibodies: a rabbit polyclonal against p-Akt (Ser473), cleaved caspase-3, or cleaved caspase-9 (all from Cell Signaling), or a mouse polyclonal against PtdIns(3,4,5)P3, followed with an appropriate IgG antibody conjugated with AlexaFluor-488, and analyzed by flow cytometry.
Western blot and immunofluorescence microscopic analyses
The antibodies used in Western blot analysis7,9 were as follows: mouse monoclonals: Smac/Diablo or cleaved poly (ADP-ribose) polymerase (PARP) from BD Biosciences; Bax, Omi/HtrA2, or β-actin, from Santa Cruz Biotechnology; KDEL from Stressgen; and protein disulfide isomerase (PDI) from Novus Biologicals; rabbit polyclonals: p-PERK (Thr980) from Novus Biologicals; SH2-containing inositol-5′-phosphatase-1 (SHIP1), p-SHIP1 (Tyr1020), Akt, p-Akt (Ser473), phosphatidylinositol 3-kinases (PI3K)-110α, PI3K-85α, eukaryotic translation initiation factor 2α (eIF2α), p-eIF2α (Ser51), from Cell Signaling; phosphatase and tensin homolog deleted on chromosome 10 (PTEN), p-PTEN (Ser370), or activating transcription factor 4 (ATF4), from Abcam; Mcl-1 and C/EBP-homologous protein (CHOP) from Santa Cruz Biotechnology; and the manganese superoxide dismutase (Mn-SOD) from Stressgen; rabbit monoclonals: GSK-3β and p-GSK-3β (Ser9) from Cell Signaling. Protein expression was quantified by densitometry analysis using Quality One, Version 4.6.5 (Bio-Rad).
For immunofluorescence analysis, BM neutrophils were placed onto glass slides by cytospin, fixed in 3.75% paraformaldehyde, and permeabilized in 0.2% Triton X-100. Permeabilized neutrophils were incubated overnight with a mouse monoclonal antibody against Bax (Cell Signaling), a mouse polyclonal antibody against pan Cadherin (Abcam) that stains plasma membrane, a rabbit polyclonal antibody against cytochrome c oxidase IV (COX IV) (Cell Signaling) that stains mitochondria, or GLUT1 (Santa Cruz Biotechnology). Then neutrophils were incubated with appropriate IgG antibody, conjugated with AlexaFluor-488 or AlexaFluor-555, mounted with an antifade, water-based mounting medium containing 4,6-diamidino-2-phenylindole (DAPI; Vector Laboratories), and visualized using a Zeiss Axiovert 200M inverted confocal microscope equipped with 40 × /1.3 NA or 63 × /1.4 NA oil objectives (Carl Zeiss MicroImaging). Images were acquired using LSM 5 acquisition software and processed using Zeiss LSM Image Browser Version 3.2.0.115 (Carl Zeiss MicroImaging).
Measurement of ROS, mitochondrial cytochrome c release, and cleaved caspase-9
Levels of cytosolic reactive oxygen species (ROS) were assessed by flow cytometry measuring the conversion of carboxy-H2DCFDA to carboxy-DCF using Image-iT Live Green Reactive Oxygen Species Detection Kit (Invitrogen).9
To measure mitochondrial cytochrome c release,23 2 × 106 neutrophils were treated for 10 minutes on ice with 100 μL phosphate-buffered saline containing 0.00005% digitonin (Sigma-Aldrich) and 0.1M KCl that selectively permeabilized plasma membranes. The digitonin-permeabilized cells were washed extensively to remove cytoplasmic cytochrome c present in apoptotic cells. Then cells were fixed with 3.75% formaldehyde, treated with 0.02% Triton X-100 to permeabilize mitochondrial membranes, incubated with a mouse monoclonal antibody against cytochrome c (BD Biosciences), washed, incubated with AlexaFluor-488-conjugated antibody, and analyzed by flow cytometry.
BM neutrophil lysates (200 μg) in RIPA lysis buffer (Thermo Scientific) containing protease inhibitor cocktails (Roche Diagnostics) were cleared of nonspecific contaminants by adding 10 μL of 50% Protein A PLUS agarose (Thermo Scientific). Cleaved caspase-9 in the supernatant was isolated by incubation with a rabbit polyclonal antibody against cleaved caspase-9 (Cell Signaling), precipitated with 20 μL Protein A PLUS agarose, and analyzed by Western blot.
In vivo G-CSF treatment and measurement of neutrophil respiratory burst, chemotaxis, calcium flux, glucose uptake, G6P, lactate, and total ATP
G6pc3−/− and control littermates were subjected to daily subcutaneous injection of recombinant mouse G-CSF at 100 μg per kg body weight for 1 or 5 days. Respiratory burst, chemotaxis, and calcium flux analysis were performed.7,9 Glucose uptake was measured by the rate of uptake of 2-DG (33 Ci/mmol, MP Biomedicals).21 Glucose uptake21 was also examined in annexin V–depleted BM neutrophils that were treated at 37°C for 15 minutes with 10−6M fMLP, or 0.8 μg/mL C5a, or 5 μg/mL CytB.
For intracellular G6P and lactate determination, 107 neutrophils in 500 μL of ice-cold phosphate-buffered saline were deproteinized using perchloric acid (14%, weight/volume).21 G6P in the deproteinized neutrophil lysate was determined by oxidation of G6P by yeast G6P dehydrogenase (Sigma-Aldrich) to 6-phosphogluconlactone accompanied by converting nicotinamide adenine dinucleotide phosphate (NADP+) to reduced NADP+ (NADPH), measured by excitation at 340 nm and emission at 450 nm in a Flexstation II Fluorometer (Molecular Devices).21 Lactate in deproteinized neutrophil lysates was analyzed using a BioVision kit, and the fluorescence intensities were measured by excitation at 535 nm and emission at 575 nm using a Flexstation II Fluorometer.
To measure total ATP, neutrophils (5 × 105) were lysed in 50 μL ATP assay buffer, centrifuged, and the supernatant (5 μL) mixed with ATP Probe, ATP Converter, and Developer Mix (BioVision kit), and incubated for 30 minutes at room temperature in the dark. The fluorescence intensity was determined by excitation at 535 nm and emission at 587 nm using a Flexstation II Fluorometer.
Statistical analysis
The unpaired t test and analysis of variance were performed using the GraphPad Prism Program Version 4 (GraphPad Software). Values were considered statistically significant at P < .05.
Results
Activation of PERK and PtdIns(3,4,5)P3/Akt pathways in G6pc3−/− BM neutrophils
In response to ER stress, GRP78 disassociates from the GRP78/PERK complex to activate PERK, which in turn phosphorylates eIF2α, leading to the expression of ATF4,10 which induces the expression of CHOP.24 In freshly isolated BM neutrophils, the expression of p-PERK (Thr980) was markedly increased in G6pc3−/− mice, compared with the controls (Figure 1A). Although the levels of eIF2α in G6pc3−/− and control neutrophils were similar, the levels of p-eIF2α (Ser51) were increased in G6pc3−/− neutrophils (Figure 1A). The production of ATF4 was also markedly increased, and the expression of CHOP was detectable only in G6pc3−/− neutrophils (Figure 1A). Together, the results suggest that the PERK-mediated ER stress signaling pathway is activated in freshly isolated BM neutrophils of G6pc3−/− mice.
The ER stress and cell survival signaling pathways in G6pc3−/− BM neutrophils. BM neutrophils were isolated from 6- to 8-week-old control (+/+) and G6pc3−/− (−/−) littermates as described in “Neutrophil isolation.” (A) Western blot analysis of protein extracts of neutrophils using antibodies against p-PERK, eIF2α, p-eIF2α, ATF4, CHOP, or β-actin. Each lane contains 50 μg protein. The relative protein levels of p-PERK, p-eIF2α, ATF4, or CHOP were quantified by densitometry of 3 or 4 separate pairs of Western blots. (B) Western blot analysis of protein extracts of neutrophils using antibodies against p-Akt, total Akt, p-SHIP1, SHIP1, PI3K-p85α, PI3K-p110α PTEN, p-PTEN, or β-actin. Each lane contains 50 μg protein. The relative protein levels of p-Akt or p-SHIP1 were quantified by densitometry of 3 or 4 separate pairs of Western blots. (C-D) Flow cytometric analysis of p-Akt (C) or PtdIns(3,4,5)P3 (D). Data are the mean ± SEM of 4 independent experiments. **P < .005. *P < .05.
The ER stress and cell survival signaling pathways in G6pc3−/− BM neutrophils. BM neutrophils were isolated from 6- to 8-week-old control (+/+) and G6pc3−/− (−/−) littermates as described in “Neutrophil isolation.” (A) Western blot analysis of protein extracts of neutrophils using antibodies against p-PERK, eIF2α, p-eIF2α, ATF4, CHOP, or β-actin. Each lane contains 50 μg protein. The relative protein levels of p-PERK, p-eIF2α, ATF4, or CHOP were quantified by densitometry of 3 or 4 separate pairs of Western blots. (B) Western blot analysis of protein extracts of neutrophils using antibodies against p-Akt, total Akt, p-SHIP1, SHIP1, PI3K-p85α, PI3K-p110α PTEN, p-PTEN, or β-actin. Each lane contains 50 μg protein. The relative protein levels of p-Akt or p-SHIP1 were quantified by densitometry of 3 or 4 separate pairs of Western blots. (C-D) Flow cytometric analysis of p-Akt (C) or PtdIns(3,4,5)P3 (D). Data are the mean ± SEM of 4 independent experiments. **P < .005. *P < .05.
In response to ER stress, the PtdIns(3,4,5)P3/Akt cell survival pathway is activated.12,13 Only plasma membrane-associated Akt can be phosphorylated and activated; membrane translocation of Akt is mediated by its association with membrane-bound PtdIns(3,4,5)P3. Western blot analysis showed that the levels of total Akt were similar between freshly isolated wild-type and G6pc3−/− BM neutrophils, but the levels of p-Akt (Ser473) were higher in G6pc3−/− neutrophils (Figure 1B). Flow cytometric analysis confirmed the increase in p-Akt (Ser473) in G6pc3−/− neutrophils (Figure 1C). Supporting this, the levels of membrane-bound and total PtdIns(3,4,5)P3 were also significantly higher in freshly isolated G6pc3−/− neutrophils compared with wild-type neutrophils (Figure 1D).
The levels of PtdIns(3,4,5)P3 can be regulated by PI3K,25 which phosphorylates PtdIns to PtdIns(3,4,5)P3, and by PTEN26 and SHIP1,27 which convert PtdIns(3,4,5)P3 to PtdIns(4,5)P2 and PtdIns(3,4)P2, respectively. Western blot analysis showed that the levels of PI3K-p110α and PI3K-p85α subunits as well as PTEN and p-PTEN (Ser370) were similar between freshly isolated control and G6pc3−/− neutrophils (Figure 1B). The levels of total SHIP1 were also unchanged, but levels of p-SHIP1 (Tyr1020), the major lipid phosphatase activity in neutrophils,27 were down-regulated in G6pc3−/− neutrophils (Figure 1B).
Oxidative stress and mitochondrial stress-mediated apoptosis in G6pc3−/− neutrophils
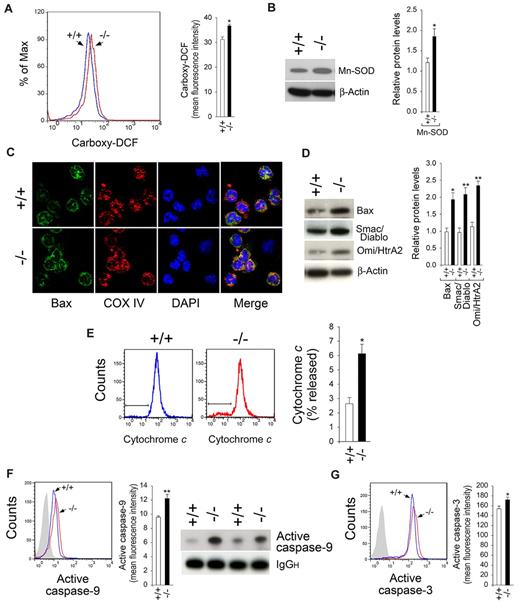
One consequence of ER stress-triggered cell death is the accumulation of ROS,28,29 which can be measured by the conversion of carboxy-H2DCFDA to the fluorescent carboxy-DCF derivative. Flow cytometric analysis (Figure 2A) showed that significantly elevated numbers of freshly isolated G6pc3−/− BM neutrophils exhibited strong fluorescent signals compared with control neutrophils, consistent with oxidative stress. The mitochondrial antioxidant enzyme Mn-SOD, which decomposes superoxide to hydrogen peroxide,30 is induced by elevated concentrations of ROS. Western blot analysis showed that expression of Mn-SOD in G6pc3−/− neutrophils was elevated over the controls (Figure 2B), supporting the presence of mitochondrial oxidative stress in freshly isolated G6pc3−/− BM neutrophils.
Oxidative stress and intrinsic mitochondrial apoptotic pathway in G6pc3−/− neutrophils. BM neutrophils were isolated from 6- to 8-week-old control (+/+) and G6pc3−/− (−/−) littermates as described in “Neutrophil isolation.” (A) Quantitative flow cytometric analysis of neutrophil carboxy-DCF staining. Data are the mean ± SEM of 4 independent experiments. (B) Western blot analysis of protein extracts of neutrophils using antibodies against Mn-SOD or β-actin. Each lane contains 50 μg protein. The relative protein levels of Mn-SOD were quantified by densitometry of 3 separate pairs of Western blots. (C) Representative confocal microscopic analysis of Bax (green fluorescence), COX IV (red fluorescence, mitochondria), and DAPI nuclei (blue fluorescence) staining (original magnification ×1000). (D) Western blot analysis of protein extracts of neutrophils using antibodies against Bax, Smac/Diablo, Omi/HtrA2, or β-actin. Each lane contains 50 μg protein. The relative protein levels of Bax, Smac/Diablo, or Omi/HtrA2 were quantified by densitometry of 3 or 4 separate pairs of Western blots. (E) Quantitative flow cytometric analysis of cytochrome c release. Data are the mean ± SEM of 3 independent experiments. (F) Flow cytometry, immunoprecipitation, and Western blot analysis of immunoprecipitates using an antibody against active caspase-9 and a horseradish peroxidase-conjugated secondary antibody. Data for flow cytometric analysis represent the mean ± SEM of 4 independent experiments. (G) Quantitative flow cytometric analysis of active caspase-3. Data are the mean ± SEM of 4 independent experiments. **P < .005. *P < .05.
Oxidative stress and intrinsic mitochondrial apoptotic pathway in G6pc3−/− neutrophils. BM neutrophils were isolated from 6- to 8-week-old control (+/+) and G6pc3−/− (−/−) littermates as described in “Neutrophil isolation.” (A) Quantitative flow cytometric analysis of neutrophil carboxy-DCF staining. Data are the mean ± SEM of 4 independent experiments. (B) Western blot analysis of protein extracts of neutrophils using antibodies against Mn-SOD or β-actin. Each lane contains 50 μg protein. The relative protein levels of Mn-SOD were quantified by densitometry of 3 separate pairs of Western blots. (C) Representative confocal microscopic analysis of Bax (green fluorescence), COX IV (red fluorescence, mitochondria), and DAPI nuclei (blue fluorescence) staining (original magnification ×1000). (D) Western blot analysis of protein extracts of neutrophils using antibodies against Bax, Smac/Diablo, Omi/HtrA2, or β-actin. Each lane contains 50 μg protein. The relative protein levels of Bax, Smac/Diablo, or Omi/HtrA2 were quantified by densitometry of 3 or 4 separate pairs of Western blots. (E) Quantitative flow cytometric analysis of cytochrome c release. Data are the mean ± SEM of 3 independent experiments. (F) Flow cytometry, immunoprecipitation, and Western blot analysis of immunoprecipitates using an antibody against active caspase-9 and a horseradish peroxidase-conjugated secondary antibody. Data for flow cytometric analysis represent the mean ± SEM of 4 independent experiments. (G) Quantitative flow cytometric analysis of active caspase-3. Data are the mean ± SEM of 4 independent experiments. **P < .005. *P < .05.
On the induction of apoptosis, Bax is oligomerized and translocated to the outer mitochondrial membrane, leading to membrane permeabilization.31,32 Confocal analysis showed that colocalization of Bax with the mitochondria-specific COX IV was found in significantly more freshly isolated G6pc3−/− BM neutrophils, compared with the controls (Figure 2C). Western blot analysis showed that the amounts of Bax proteins were increased in G6pc3−/− neutrophils (Figure 2D).
The Bax-mediated mitochondrial membrane permeabilization leads to the release of proapoptotic effectors, including cytochrome c, Smac/Diablo, and Omi/HtrA2 from the mitochondrial intermembrane space to the cytoplasm.33,34 Flow cytometric analysis showed that the release of cytochrome c into the cytosol was increased in freshly isolated G6pc3−/− BM neutrophils, compared with the controls (Figure 2E). Western blot analysis also showed that G6pc3−/− neutrophils produced increased levels of Smac/Diablo and Omi/HtrA2 (Figure 2D). The mitochondrial, stress-induced route of apoptosis involves the activation of caspase-9.35 Flow cytometric analysis showed that the levels of activated caspase-9 were increased in freshly isolated G6pc3−/− BM neutrophils, compared with the controls (Figure 2F). Immunoprecipitation and Western blot analysis confirmed the increase in the amounts of active caspase-9 proteins in G6pc3−/− neutrophils (Figure 2F). Confirming an earlier study,21 the levels of active caspase-3 were also increased in freshly isolated G6pc3−/− BM neutrophils (Figure 2G).
G-CSF can delay, but not prevent, accelerated spontaneous apoptotic death of neutrophils from G6pc3−/− mice
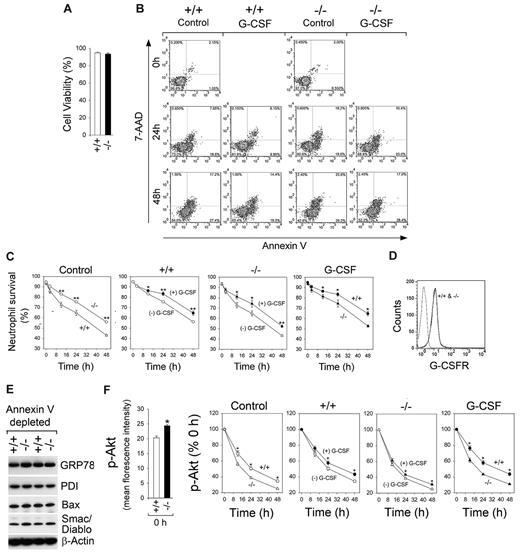
To assess the rates of spontaneous neutrophil apoptosis, annexin V–depleted control and G6pc3−/− BM neutrophils of similar viability (Figure 3A) were incubated in standard medium containing 6.2mM glucose for extended periods of time. The number of viable neutrophils [annexin V (−)/7-AAD (−)] was quantified over time (Figure 3B-C). Wild-type neutrophils underwent apoptosis during in vitro culturing with viable counts decreasing from 95.0% to 75.7% and 56.2%, respectively, after 24 and 48 hours of culture (Figure 3C). The G6pc3−/− neutrophils exhibited an enhanced rate of apoptosis in vitro with viable counts, decreasing from 93.4% to 64.8% and 43.2%, respectively, after 24 and 48 hours of culture (Figure 3C).
G-CSF can delay but not prevent apoptotic death of G6pc3−/− BM neutrophils cultured in vitro. The annexin V–depleted BM neutrophils were isolated from 6- to 8-week-old unaffected (+/+, ○, ●) and G6pc3−/− (−/−, Δ, ▴) littermates as described in “Neutrophil isolation.” (A) Neutrophil viability. (B) Representative flow cytometric analysis of neutrophil survival. (C) Quantitative analysis of neutrophil survival in untreated wild-type (○, ●) and G6pc3−/− (Δ, ▴) mice in the absence (○, Δ) or presence (●, ▴) of G-CSF. Data are the mean ± SEM of 4 independent experiments. (D) Flow cytometric analysis of G-CSFR. (E) Western blot analysis of protein extracts of neutrophils using antibodies against GRP78, PDI, Bax, Smac/Diablo, or β-actin. Each lane contains 50 μg protein. (F) Quantitative flow cytometric analysis of p-Akt in neutrophils of untreated wild-type (○, ●) and G6pc3−/− (Δ, ▴) mice in the absence (○, Δ) or presence (●, ▴) of G-CSF. Data are the mean ± SEM of 3 independent experiments. **P < .005. *P < .05.
G-CSF can delay but not prevent apoptotic death of G6pc3−/− BM neutrophils cultured in vitro. The annexin V–depleted BM neutrophils were isolated from 6- to 8-week-old unaffected (+/+, ○, ●) and G6pc3−/− (−/−, Δ, ▴) littermates as described in “Neutrophil isolation.” (A) Neutrophil viability. (B) Representative flow cytometric analysis of neutrophil survival. (C) Quantitative analysis of neutrophil survival in untreated wild-type (○, ●) and G6pc3−/− (Δ, ▴) mice in the absence (○, Δ) or presence (●, ▴) of G-CSF. Data are the mean ± SEM of 4 independent experiments. (D) Flow cytometric analysis of G-CSFR. (E) Western blot analysis of protein extracts of neutrophils using antibodies against GRP78, PDI, Bax, Smac/Diablo, or β-actin. Each lane contains 50 μg protein. (F) Quantitative flow cytometric analysis of p-Akt in neutrophils of untreated wild-type (○, ●) and G6pc3−/− (Δ, ▴) mice in the absence (○, Δ) or presence (●, ▴) of G-CSF. Data are the mean ± SEM of 3 independent experiments. **P < .005. *P < .05.
Neutropenia in G6PC3-deficient patients has been successfully treated by G-CSF therapy.8,17 Consistent with this, we show that annexin V–depleted wild-type and G6pc3−/− BM neutrophils expressed similar levels of the G-CSF receptor (G-CSFR)36 (Figure 3D). Nonapoptotic control and G6pc3−/− BM neutrophils also expressed similar levels of molecular chaperons (GRP78 and protein disulfide isomerase) and apoptotic mediators (Bax and Smac/Diablo) (Figure 3E). Moreover, the levels of active caspase-9 in nonapoptotic control and G6pc3−/− BM neutrophils were 0.72% and 1.05%, respectively, and active caspase-3 were 3.1% and 3.9%, respectively. Together, the results showed that annexin V–depleted control and G6pc3−/− neutrophils were of similar biologic viability.
The survival of nonapoptotic control and G6pc3−/− BM neutrophils was examined after culturing in vitro for extended times in standard medium containing mouse G-CSF. G-CSF delayed the induction of apoptosis in wild-type and G6pc3−/− neutrophils (Figure 3B-C). Despite delaying apoptosis, neutrophils from untreated G6pc3−/− mice cultured in standard medium in the presence of G-CSF continued to exhibit accelerated rates of apoptotic death in vitro, compared with controls (Figure 3B-C).
Freshly isolated (Figure 1B) and annexin V–depleted (Figure 3F) neutrophils from untreated G6pc3−/− mice expressed significantly higher levels of p-Akt, compared with controls. The levels of p-Akt declined in a time-dependent fashion in annexin V–depleted wild-type and G6pc3−/− neutrophils undergoing spontaneous apoptosis after culturing in standard medium (Figure 3F). In vitro G-CSF treatment delayed the decline in p-Akt in both neutrophil populations, but the decline in p-Akt remained faster in G6pc3−/− neutrophils in the presence of G-CSF (Figure 3F).
Effects of limiting glucose, fMLP, C5a, CytB, and 2-DG on apoptosis of G6pc3−/− neutrophils
The survival of nonapoptotic control and G6pc3−/− BM neutrophils was examined after in vitro culturing in limited glucose (0.6mM) compared with culturing in standard medium containing 6.2mM glucose (Figure 4A). In limited glucose, the rates of apoptosis of both wild-type and G6pc3−/− neutrophils increased but was more pronounced for wild-type neutrophils, leading to similar survival profiles for both neutrophil populations (Figure 4A). G-CSF delayed the induction of apoptosis in wild-type and G6pc3−/− neutrophils in limited glucose, and their survival profiles remained similar in the presence of G-CSF (Figure 4A).
Effects of limiting glucose, fMLP, C5a, CytB, and 2-DG on apoptotic death of G6pc3−/− BM neutrophils cultured in vitro. The annexin V–depleted BM neutrophils were isolated from 6- to 8-week-old unaffected (+/+) and G6pc3−/− (−/−) littermates as described in “Neutrophil isolation.” (A) Quantitative analysis of neutrophil survival in untreated wild-type (○, ●, □, ■) and G6pc3−/− (Δ, ▴, ▿, ▾) mice in 6.2mM glucose (○, ●, Δ, ▴,) or 0.6mM glucose (□, ■, ▿, ▾)-containing medium in the absence (○, □, Δ, ▿) or presence (●, ■, ▴, ▾) of G-CSF. Data are the mean ± SEM of 4 independent experiments. (B) Effect of fMLP, C5a, or CytB on neutrophil 2-DG uptake. Glucose uptake was examined in annexin V–depleted BM neutrophils that were treated at 37°C for 15 minutes with 10−6M fMLP, 0.8 μg/mL C5a, or 5 μg/mL CytB. Data are the mean ± SEM of 3 independent experiments. (C) Quantitative analysis of neutrophil survival in untreated wild-type (○, ×, □, ●) and G6pc3−/− (Δ, ▿, ★, ▴) mice in 6.2mM glucose-containing medium in the presence of 10−6M fMLP (×, ▿), 0.8 μg/mL C5a (□, ★), or 5 μg/mL CytB (●, ▴). (D) Quantitative analysis of neutrophil survival in untreated wild-type (○, ●, □, ■) and G6pc3−/− (Δ, ▴, ▿, ▾) mice in 6.2mM glucose-containing medium in the absence (○, Δ, ●, ▴) or presence of 1mM 2-DG (□, ■, ▿, ▾), in the presence of G-CSF (●, ▴), or 2-DG/G-CSF (■, ▾). **P < .005. *P < .05.
Effects of limiting glucose, fMLP, C5a, CytB, and 2-DG on apoptotic death of G6pc3−/− BM neutrophils cultured in vitro. The annexin V–depleted BM neutrophils were isolated from 6- to 8-week-old unaffected (+/+) and G6pc3−/− (−/−) littermates as described in “Neutrophil isolation.” (A) Quantitative analysis of neutrophil survival in untreated wild-type (○, ●, □, ■) and G6pc3−/− (Δ, ▴, ▿, ▾) mice in 6.2mM glucose (○, ●, Δ, ▴,) or 0.6mM glucose (□, ■, ▿, ▾)-containing medium in the absence (○, □, Δ, ▿) or presence (●, ■, ▴, ▾) of G-CSF. Data are the mean ± SEM of 4 independent experiments. (B) Effect of fMLP, C5a, or CytB on neutrophil 2-DG uptake. Glucose uptake was examined in annexin V–depleted BM neutrophils that were treated at 37°C for 15 minutes with 10−6M fMLP, 0.8 μg/mL C5a, or 5 μg/mL CytB. Data are the mean ± SEM of 3 independent experiments. (C) Quantitative analysis of neutrophil survival in untreated wild-type (○, ×, □, ●) and G6pc3−/− (Δ, ▿, ★, ▴) mice in 6.2mM glucose-containing medium in the presence of 10−6M fMLP (×, ▿), 0.8 μg/mL C5a (□, ★), or 5 μg/mL CytB (●, ▴). (D) Quantitative analysis of neutrophil survival in untreated wild-type (○, ●, □, ■) and G6pc3−/− (Δ, ▴, ▿, ▾) mice in 6.2mM glucose-containing medium in the absence (○, Δ, ●, ▴) or presence of 1mM 2-DG (□, ■, ▿, ▾), in the presence of G-CSF (●, ▴), or 2-DG/G-CSF (■, ▾). **P < .005. *P < .05.
We then examined the survival of nonapoptotic control and G6pc3−/− BM neutrophils after culturing in vitro in standard medium in the presence of glucose uptake stimulators fMLP37 or C5a38 and the glucose uptake inhibitor CytB39 (Figure 4B). fMLP or C5a did not affect the survival of control or G6pc3−/− BM neutrophils, and G6pc3−/− neutrophils continued to exhibit an enhanced rate of apoptosis, compared with controls (Figure 4C). In contrast, CytB stimulated apoptosis of both control and G6pc3−/− BM neutrophils, but G6pc3−/− neutrophils continued to exhibit an enhanced rate of apoptosis, compared with controls (Figure 4C).
The survival of nonapoptotic control and G6pc3−/− BM neutrophils in standard medium was further examined in the presence of 1mM 2-DG, a glucose analog that is known to contribute to cell death.40 As expected, 2-DG enhanced the rates of apoptosis of control and G6pc3−/− neutrophils both in the absence and presence of G-CSF, and G6pc3−/− neutrophils continued have accelerated apoptotic death rates, compared with the controls (Figure 4D).
G-CSF delays G6pc3−/− neutrophil apoptosis by modulating apoptotic mediators
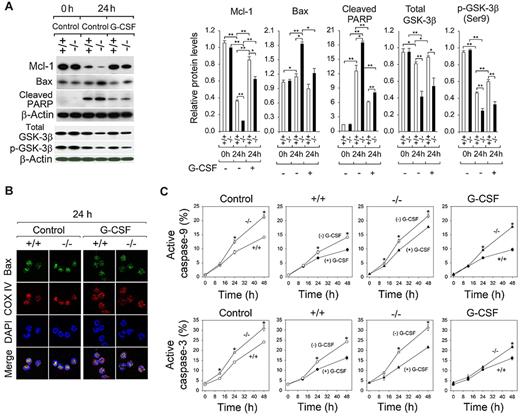
At 0 hours, similar high levels of the antiapoptotic Mcl-1 protein were observed in annexin V–depleted BM neutrophils from untreated wild-type and G6pc3−/− mice (Figure 5A). The levels of Mcl-1 decreased markedly after 24 hours in culture, and G-CSF prevented the decline in both neutrophil populations (Figure 5A). Consistent with an enhanced rate of apoptosis of G6pc3−/− neutrophils, levels of Mcl-1 in G6pc3−/− neutrophils were consistently lower than those in wild-type neutrophils both in the absence and presence of G-CSF (Figure 5A). Studies have shown that inactivation of GSK-3β leads to Mcl-1 stabilization.41,42 At 0 hours, similar levels of total GSK-3β and the inactive p-GSK-3β (Ser9) were observed in annexin V–depleted BM neutrophils from untreated wild-type and G6pc3−/− mice (Figure 5A). After 24 hours in culture, the levels of total GSK-3β and p-GSK-3β were decreased in wild-type and G6pc3−/− neutrophils but were more prominent for G6pc3−/− neutrophils, resulting in reduced levels of both forms of GSK-3β in G6pc3−/− neutrophils (Figure 5A). The levels of total GSK-3β and p-GSK-3β in control and G6pc3−/− neutrophils were not significantly altered by G-CSF treatment (Figure 5A), suggesting that GSK-3β plays little role in modulation of neutrophil apoptosis by G-CSF.
G-CSF delays G6pc3−/− neutrophil apoptosis by modulating apoptotic mediators. The annexin V–depleted BM neutrophils were isolated from 6- to 8-week-old unaffected (+/+, ○, ●) and G6pc3−/− (−/−, Δ, ▴) littermates as described in “Neutrophil isolation.” (A) Western blot analysis of protein extracts of neutrophils using antibodies against Mcl-1, Bax, cleaved PARP, total GSK-3β, p-GSK-3β (Ser9), or β-actin. Each lane contains 50 μg protein. The relative protein levels of Mcl-1, Bax, cleaved PARP, total GSK-3β, or p-GSK-3β (Ser9) were quantified by densitometry of 3 or 4 separate pairs of Western blots. (B) Representative confocal microscopic analysis of Bax (green fluorescence), COX IV (red fluorescence, mitochondria), and DAPI nuclei (blue fluorescence) staining (original magnification ×1000). (C) Quantitative flow cytometric analysis of active caspase-9 and caspase-3 in neutrophils of untreated wild-type (○, ●) and G6pc3−/− (Δ, ▴) mice in the absence (○, Δ) or presence (●, ▴) of G-CSF. Data are the mean ± SEM of 3 independent experiments. **P < .005. *P < .05.
G-CSF delays G6pc3−/− neutrophil apoptosis by modulating apoptotic mediators. The annexin V–depleted BM neutrophils were isolated from 6- to 8-week-old unaffected (+/+, ○, ●) and G6pc3−/− (−/−, Δ, ▴) littermates as described in “Neutrophil isolation.” (A) Western blot analysis of protein extracts of neutrophils using antibodies against Mcl-1, Bax, cleaved PARP, total GSK-3β, p-GSK-3β (Ser9), or β-actin. Each lane contains 50 μg protein. The relative protein levels of Mcl-1, Bax, cleaved PARP, total GSK-3β, or p-GSK-3β (Ser9) were quantified by densitometry of 3 or 4 separate pairs of Western blots. (B) Representative confocal microscopic analysis of Bax (green fluorescence), COX IV (red fluorescence, mitochondria), and DAPI nuclei (blue fluorescence) staining (original magnification ×1000). (C) Quantitative flow cytometric analysis of active caspase-9 and caspase-3 in neutrophils of untreated wild-type (○, ●) and G6pc3−/− (Δ, ▴) mice in the absence (○, Δ) or presence (●, ▴) of G-CSF. Data are the mean ± SEM of 3 independent experiments. **P < .005. *P < .05.
The levels of Bax were increased in neutrophils of untreated G6pc3−/− mice after 24 hours in culture (Figure 5A). Supporting this, confocal microscopy analysis showed that, after 24 hours in culture, the levels of mitochondria-associated Bax were significantly increased in annexin V–depleted G6pc3−/− BM neutrophils (Figure 5B). G-CSF prevented the increase in the mitochondria-associated Bax in both neutrophil populations. In the presence of G-CSF, the levels of Bax protein were slightly higher in G6pc3−/− neutrophils than control neutrophils, but the difference was not statistically significant (Figure 5A).
Consistent with enhanced rates of neutrophil apoptosis of untreated G6pc3−/− mice, levels of active caspase-9 and caspase-3 were consistently higher in G6pc3−/− neutrophils than those in control neutrophils during culturing in vitro (Figure 5C). In vitro G-CSF treatment prevented the increases in activated caspase-9 and caspase-3 in both neutrophil populations (Figure 5C). Again, in the presence of G-CSF, levels of activated caspases remained higher in G6pc3−/− neutrophils than controls (Figure 5C).
The cleavage of PARP by caspases is one of the most used diagnostic markers for apoptosis.43 At 0 hours, very low levels of cleaved PARP were observed in annexin V–depleted BM neutrophils from untreated control and G6pc3−/− mice (Figure 5A). The amounts of cleaved PARP increased markedly in both neutrophil populations after 24 hours in culture, and in vitro G-CSF treatment prevented the increase in control and G6pc3−/− BM neutrophils (Figure 5A). Again, the levels of cleaved PARP remained higher in G6pc3−/− neutrophils. Taken together, our results show that, in untreated mice, G-CSF could delay apoptosis of in vitro cultured G6pc3−/− neutrophils but could not prevent their accelerated death.
In vivo G-CSF therapy corrects neutropenia and neutrophil dysfunction in G6pc3−/− mice
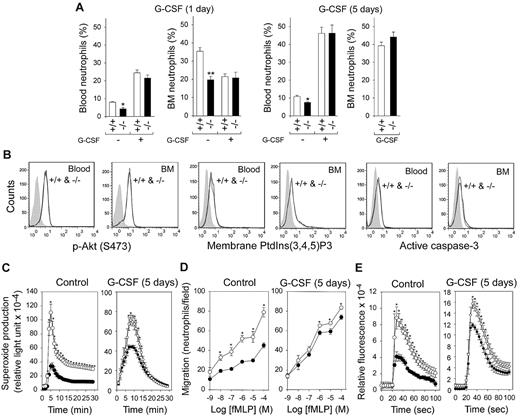
Twenty-four hours after a single injection of G-CSF markedly increased neutrophil counts in control and G6pc3−/− mice, neutropenia was relieved in G6pc3−/− mice (Figure 6A) but G6pc3−/− neutrophil functionality was not improved (data not shown).
In vivo G-CSF therapy corrects neutropenia and improves neutrophil function in G6pc3−/− mice. G6pc3−/− (−/−) and control (+/+) littermates were treated with recombinant mouse G-CSF for 1 or 5 days. (A) Blood and BM neutrophil counts in untreated and G-CSF–treated mice. (B) Representative flow cytometric analysis of neutrophil p-Akt, membrane-bound PtdIns(3,4,5)P3, and active caspase-3 in 5-day G-CSF–treated control and G6pc3−/− mice. (C) Neutrophil respiratory burst activity in response to 200 ng/mL of phorbol myristate acetate in untreated and 5-day G-CSF–treated control (○) and G6pc3−/− (●) mice. (D) Neutrophil concentration-dependent chemotaxis in response to fMLP in untreated and 5-day G-CSF–treated control (○) and G6pc3−/− (●) mice. (E) Neutrophil calcium flux in response to 10−6M of fMLP in untreated and 5-day G-CSF–treated control (○) and G6pc3−/− (●) mice. Data are the mean ± SEM of 3 (untreated) or 6 (G-CSF–treated) independent experiments. *P < .05.
In vivo G-CSF therapy corrects neutropenia and improves neutrophil function in G6pc3−/− mice. G6pc3−/− (−/−) and control (+/+) littermates were treated with recombinant mouse G-CSF for 1 or 5 days. (A) Blood and BM neutrophil counts in untreated and G-CSF–treated mice. (B) Representative flow cytometric analysis of neutrophil p-Akt, membrane-bound PtdIns(3,4,5)P3, and active caspase-3 in 5-day G-CSF–treated control and G6pc3−/− mice. (C) Neutrophil respiratory burst activity in response to 200 ng/mL of phorbol myristate acetate in untreated and 5-day G-CSF–treated control (○) and G6pc3−/− (●) mice. (D) Neutrophil concentration-dependent chemotaxis in response to fMLP in untreated and 5-day G-CSF–treated control (○) and G6pc3−/− (●) mice. (E) Neutrophil calcium flux in response to 10−6M of fMLP in untreated and 5-day G-CSF–treated control (○) and G6pc3−/− (●) mice. Data are the mean ± SEM of 3 (untreated) or 6 (G-CSF–treated) independent experiments. *P < .05.
A series of 5 consecutive daily injections of G-CSF in G6pc3−/− mice both corrected neutropenia (Figure 6A) and normalized levels of p-Akt, PtdIns(3,4,5)P3, and active caspase-3 in blood and BM neutrophils (Figure 6B). Importantly, extended in vivo G-CSF treatment markedly improved respiratory burst, chemotaxis, and calcium flux activities in G6pc3−/− neutrophils (Figure 6C).
In vivo G-CSF therapy improves energy homeostasis in G6pc3−/− neutrophils
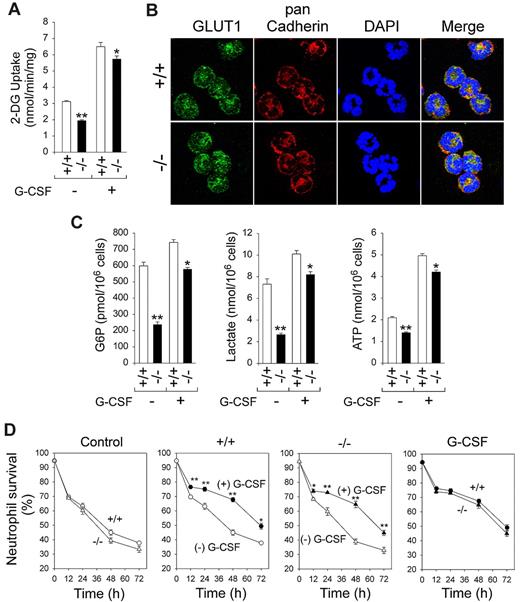
We have previously shown that G6PC3-deficient neutrophils exhibit impaired glucose uptake and reduced intracellular levels of G6P, lactate, and ATP.21 We hypothesized that G-CSF corrects neutrophil function in G6pc3−/− mice by enhancing neutrophil glucose transport and energy homeostasis. Although 2-DG uptake in untreated wild-type neutrophils was 3.12 ± 0.06 nmol/min per milligram, the 5-day G-CSF therapy increased 2-DG uptake in these neutrophils by 2.1-fold to 6.50 ± 0.26 nmol/min per milligram (Figure 7A). The 5-day G-CSF therapy also markedly increased 2-DG uptake in G6pc3−/− neutrophils from an untreated level of 1.94 ± 0.06 to 5.73 ± 0.20 nmol/min per milligram (Figure 7A). Supporting this, confocal microscopic analysis showed that the expression of membrane associated GLUT1 became similar between control and G6pc3−/− neutrophils (Figure 7B). As expected, after 5-day G-CSF treatment, intracellular levels of G6P increased from 30% to 78%, levels of lactate increased from 36% to 82%, and levels of ATP increased from 68% to 85%, relative to control neutrophils of untreated G6pc3−/− mice (Figure 7C).
In vivo G-CSF therapy increases neutrophil 2-DG uptake, the expression of GLUT1, and intracellular G6P, lactate, and ATP levels. BM neutrophils were isolated from 6- to 8-week-old control (+/+) and G6pc3−/− (−/−) littermates after 5-day G-CSF therapy. (A) Neutrophil 2-DG uptake in untreated and 5-day G-CSF–treated control and G6pc3−/− mice. Data are the mean ± SEM of 3 (untreated) or 6 (G-CSF–treated) independent experiments. (B) Representative immunofluorescence of neutrophil GLUT1 staining (green fluorescence), pan Cadherin membrane staining (red fluorescence), and DAPI nuclei staining (blue fluorescence) (original magnification ×1000). (C) Neutrophil G6P, lactate, and ATP levels in untreated and 5-day G-CSF–treated control and G6pc3−/− mice. Data are the mean ± SEM of 3 (untreated) or 6 (G-CSF–treated) independent experiments. (D) Quantitative flow cytometric analysis of survival of in vitro cultured neutrophils isolated from 5-day G-CSF–treated wild-type (○, ●) and G6pc3−/− (Δ, ▴) mice in the absence (○, Δ) or presence (●, ▴) of G-CSF. Data are the mean ± SEM of 4 independent experiments. **P < .005. *P < .05.
In vivo G-CSF therapy increases neutrophil 2-DG uptake, the expression of GLUT1, and intracellular G6P, lactate, and ATP levels. BM neutrophils were isolated from 6- to 8-week-old control (+/+) and G6pc3−/− (−/−) littermates after 5-day G-CSF therapy. (A) Neutrophil 2-DG uptake in untreated and 5-day G-CSF–treated control and G6pc3−/− mice. Data are the mean ± SEM of 3 (untreated) or 6 (G-CSF–treated) independent experiments. (B) Representative immunofluorescence of neutrophil GLUT1 staining (green fluorescence), pan Cadherin membrane staining (red fluorescence), and DAPI nuclei staining (blue fluorescence) (original magnification ×1000). (C) Neutrophil G6P, lactate, and ATP levels in untreated and 5-day G-CSF–treated control and G6pc3−/− mice. Data are the mean ± SEM of 3 (untreated) or 6 (G-CSF–treated) independent experiments. (D) Quantitative flow cytometric analysis of survival of in vitro cultured neutrophils isolated from 5-day G-CSF–treated wild-type (○, ●) and G6pc3−/− (Δ, ▴) mice in the absence (○, Δ) or presence (●, ▴) of G-CSF. Data are the mean ± SEM of 4 independent experiments. **P < .005. *P < .05.
G-CSF also delayed the induction of apoptosis in neutrophils of 5-day in vivo G-CSF–treated wild-type and G6pc3−/− mice during culture in vitro (Figure 7D). Unlike neutrophils of untreated mice, neutrophils from 5-day in vivo G-CSF–treated control and G6pc3−/− mice exhibited similar apoptotic death profiles in the absence and presence of G-CSF (Figure 7D).
Discussion
The G6Pase-β/G6PT complex that transports cytoplasmic G6P into the ER lumen then hydrolyzes it into glucose and phosphate is one of the primary routes of G6P metabolism in neutrophils.1,2,7,21 G6Pase-β and G6PT are functionally coupled, and a deficiency in either leads to a common myeloid phenotype, characterized by neutropenia and neutrophil dysfunction.4,5,7,9,21 Both G6PC3-deficient7,8,21 and GSD-Ib6,9 neutrophils exhibit enhanced ER stress and apoptosis. In this study, we show that one pathway that mediates ER stress in G6pc3−/− neutrophils is the PERK signaling10,11 and that G6pc3−/− neutrophils undergoing ER stress also activate the PtdIns(3,4,5)P3/Akt cell survival pathway.12,13 We have previously shown that the intrinsic mitochondrial stress pathway mediates apoptosis of murine G6pt−/− neutrophils.9 In this study, we show that a similar pathway mediates apoptotic death of G6pc3−/− neutrophils. Moreover, neutrophils from untreated G6pc3−/− mice exhibit accelerated rates of spontaneous apoptosis in vitro, accompanied by down-regulation of p-Akt. G-CSF delays the apoptosis, but G6pc3−/− neutrophils continue to display rates of spontaneous apoptotic death greater than those of similarly treated control neutrophils. A 5-day in vivo G-CSF treatment of G6pc3−/− mice corrects neutropenia, improves neutrophil function, stimulates neutrophil glucose uptake, and increases intracellular levels of neutrophil G6P, lactate, and ATP. Previously, we showed that G6Pase-β is essential for neutrophil energy homeostasis and functionality.21 In this study, we extend this to show that endogenous glucose production in the ER by G6Pase-β is also essential for neutrophil survival in vitro and that G-CSF delays G6pc3−/− neutrophil apoptosis by modulating apoptotic modulator. We also show that in vivo G-CSF therapy stimulates glucose uptake in G6pc3−/− neutrophils, leading to improved energy homeostasis and functionality.
Limiting glucose in vitro from a standard concentration of 6.2mM to 0.6mM increases apoptosis of both wild-type and G6pc3−/− neutrophils, but the increase is significantly greater for wild-type neutrophils, resulting in near-identical survival profiles for both neutrophil populations. We have shown that G6Pase-β is required for GLUT1 expression and membrane translocation in neutrophils, and G6Pase-β-deficient neutrophils exhibit impaired glucose uptake.21 The near-identical survival rates of control and G6pc3−/− neutrophils in the presence of low levels of exogenous glucose both in the absence and presence of G-CSF suggest that, when glucose is limited, wild-type neutrophils are in the same position as the glucose-uptake impaired G6pc3−/− neutrophils. Perhaps a clearer way of stating this is that limiting glucose available to wild-type neutrophils cultured in vitro, under conditions where glucose is the sole energy source, markedly reduces intracellular levels of G6P, ATP, and lactate, which in turn impair GLUT1 translocation and glucose uptake.21 This leads to enhanced apoptosis. On the other hand, intracellular levels of G6P, ATP, and lactate are inherently low in G6pc3−/− neutrophils because of the reduced activity of GLUT1.21 Therefore, until the external glucose is lowered to an extent that matches the GLUT1 transporter activity, limiting exogenous glucose will have little impact. Supporting this, the differential in vitro survival rates were unchanged by altering glucose uptake or treatment with 2-DG that enhances the rates of neutrophil apoptosis.40 G-CSF improves the survival of both neutrophil populations in both low and high glucose, suggesting that G-CSF delays neutrophil apoptosis by modulating apoptotic modulators.
Granulocyte-macrophage CSF has been shown to stimulate cellular glucose uptake by modulating the GLUTs,44 but the role of G-CSF in this has not been firmly established. We hypothesized that G-CSF improves G6pc3−/− neutrophil function by stimulating cellular glucose uptake and enhancing energy homeostasis. An in vivo G-CSF therapy stimulates GLUT1 membrane translocation and glucose uptake in both wild-type and G6pc3−/− neutrophils. Accordingly, cellular levels of G6P, lactate, and ATP were elevated in both neutrophil populations. The increases were more pronounced in G6pc3−/− neutrophils with the rates of 2-DG uptake increasing from 62% of the control levels in untreated to 88% in G-CSF treated (Figure 7A) mice. Likewise, levels of G6P, lactate, and ATP in G6pc3−/− neutrophils increased from 40%, 36%, and 68%, respectively, of the control levels in untreated mice to 78%, 82%, and 85%, respectively, of the control levels in G-CSF–treated (Figure 7C) mice. Consistent with this, in vivo G-CSF therapy markedly enhanced respiratory burst, chemotaxis, and calcium flux activities in G6pc3−/− neutrophils. Although G-CSF failed to prevent accelerated apoptotic death of in vitro cultured neutrophils from untreated G6pc3−/− mice, the spontaneous apoptotic death profiles of neutrophils from G-CSF–treated wild-type and G6pc3−/− mice were indistinguishable. The ability of G-CSF therapy to improve neutrophil function provides one explanation why Boztug et al8 failed to detect neutrophil dysfunction, whereas McDermott et al showed impaired neutrophil respiratory burst activity in human G6PC3-deficient patients.17
G-CSF therapy improves neutropenia in both G6PC3-deficient8,17 and GSD-Ib15,16,22 patients but does not prevent apoptotic death of human GSD-Ib neutrophils cultured in vitro.6 This suggests that, despite the similarity of the pathways leading to ER stress and apoptosis in GSD-Ib and G6PC3-deficient neutrophils, G-CSF may have differential effects. This could reflect G-CSFR expression. Although G6pc3−/− neutrophils express wild-type levels of this receptor (Figure 3D), the levels of G-CSFR in G6PT-deficient neutrophils have not been examined.6 An alternative, nonexclusive, explanation might be that despite the functional codependence of G6PT and G6Pase-β, neutrophils deficient in G6PT, a G6P transport defect, are functionally different from neutrophils lacking G6Pase-β, a G6P hydrolysis defect. Indeed, endoluminal G6P can also be converted to 6-phosphogluconolactone by the ER enzyme hexose-6-phosphate dehydrogenase.45,46 The resulting NADPH generated by this pathway within the ER is important for in vivo function of 11β-hydroxysteroid dehydrogenase-1, an ER-associated oxoreductase that generates cortisol/corticosterone to modulate immune responses.47 To this end, it would be interesting to examine hexose-6-phosphate dehydrogenase-deficient mice46 to assess whether a NADPH deficiency in the ER desensitizes neutrophils to G-CSF. Notably, the difference does not appear to be the result of a lack of serum G-CSF. Serum G-CSF levels were similar between wild-type and G6pc3−/− mice17 but markedly elevated in GSD-Ib patients and mice.48
Neutrophils express the antiapoptotic Mcl-1 protein, and cellular levels of Mcl-1 in human neutrophils correlate closely with their survival kinetics.49 It has been suggested that GSK-3β, which negatively regulates Mcl-1 levels,41,42 plays a role in neutrophil apoptosis in G6PC3 deficiency.8 We show that, after in vitro culturing, the levels of total GSK-3β and p-GSK-3β were lower in G6pc3−/− neutrophils compared with wild-type neutrophils. Although G-CSF increased the levels of Mcl-1 in both wild-type and G6pc3−/− neutrophils cultured in vitro, the levels of total GSK-3β and p-GSK-3β were not significantly altered, suggesting that GSK-3β plays little role in modulation of neutrophil apoptosis by G-CSF.
During neutrophil apoptosis, oligomerization of Bax at the mitochondrial membrane forms large pores, leading to the release of proapoptotic effectors from the mitochondrial intermembrane space to the cytoplasm.31,32 The cytosolic cytochrome c mediates the formation of a complex between apoptotic protease activating factor-1 and procaspase-9, resulting in caspase-9 activation, which in turn activates downstream effector caspases that ultimately lead to cell death.35 In G6pc3−/− neutrophils, levels of Bax are elevated in the mitochondrial membrane over control neutrophils. Accordingly, the release of cytochrome c into the cytosol of the G6pc3−/− neutrophils increased compared with control neutrophils, leading to increased levels of activated caspase-9 and caspase-3. G-CSF delays apoptotic death of in vitro cultured neutrophils from untreated G6pc3−/− mice. This delay is mediated by modulating apoptotic mediators: the reduced rate of the decline in Mcl-1, the inhibition of Bax translocation to the mitochondria, the blocking of activation of caspase-9/caspase-3, the prevention of the increase in cleaved PARP, and the delay in the decline in p-Akt.
In conclusion, we have elucidated the potential mechanism underlying neutrophil apoptosis in murine G6PC3 deficiency. We show that PERK signaling is one ER stress pathway activated in G6pc3−/− neutrophils and apoptosis is mediated, in part, by the mitochondrial stress pathway. G6pc3−/− neutrophils undergoing ER stress also activate the PtdIns(3,4,5)P3/Akt cell survival signaling pathway. G-CSF delays apoptosis of cultured G6pc3−/− neutrophils by modulating apoptotic mediators. We have further elucidated the potential mechanism underlying correction of G6pc3−/− neutrophil dysfunction by G-CSF. We show that in vivo G-CSF therapy increases glucose uptake, normalizes energy homeostasis, and improves functionality in G6pc3−/− neutrophils.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Intramural Research Programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: H.S.J. designed and performed the research, analyzed data, and wrote the paper; Y.M.L. and K.D.S. performed the research and analyzed data; B.C.M. analyzed data and wrote the paper; and J.Y.C. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Janice Y. Chou, National Institutes of Health, 10 Center Dr, Bldg 10, Rm 9D42, Bethesda, MD 20892-1830; e-mail: chouja@mail.nih.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal