Abstract
Recent data suggest that CD8+ T-cell effector activity is an important component in the control of HIV replication in elite controllers (ECs). One critical element of CD8+ T-cell effector function and differentiation is the T-box transcription factor T-bet. In the present study, we assessed T-bet expression, together with the effector proteins perforin, granzyme A (Grz A), granzyme B (Grz B), and granulysin, in HIV-specific CD8+ T cells from ECs (n = 20), chronically infected progressors (CPs; n = 18), and highly active antiretroviral therapy (HAART)–suppressed individuals (n = 19). Compared with the other cohort groups, HIV-specific CD8+ T cells among ECs demonstrated a superior ability to express perforin and Grz B, but with no detectable difference in the levels of Grz A or granulysin. We also observed higher levels of T-bet in HIV-specific CD8+ T cells from ECs, with an ensuing positive correlation between T-bet and levels of both perforin and Grz B. Moreover, HIV-specific CD8+ T cells in ECs up-regulated T-bet to a greater extent than CPs after in vitro expansion, with concomitant up-regulation of perforin and Grz B. These results suggest that T-bet may play an important role in driving effector function, and its modulation may lead to enhanced effector activity against HIV.
Introduction
HIV infection is typically associated with high viral loads and steadily declining CD4+ T-cell counts until eventual immune system collapse with the onset of AIDS. However, a rare subset of HIV-infected individuals termed “elite controllers” (ECs) can spontaneously control viral load to extremely low levels without the intervention of antiretroviral therapy. Understanding the mechanism(s) by which ECs are able to control HIV replication is an area of intense research interest that may provide necessary insights for the development of vaccines and therapeutics to combat HIV.1,2
Recent data have shown that HIV-specific CD8+ T cells from ECs have enhanced cytotoxic function compared with progressors. CD8+ T cells from ECs displayed a superior ability to suppress the replication of HIV in autologous CD4+ T cells during extended culture.3,4 CD8+ T cells from ECs that were expanded in vitro for 6 days after HIV-specific stimulation demonstrated enhanced proliferation and up-regulation of perforin and granzyme B (Grz B).5,6 The up-regulation of these cytotoxic, granule-resident proteins during culture translated into a greater capacity to induce target-cell death on a per-cell basis.6 In addition, ECs express higher levels of perforin immediately after antigen recognition, resulting in a greater ex vivo cytotoxic potential.7 These findings suggest that CD8+ T cells play a critical role in the control of HIV replication, particularly within ECs.
CD8+ T cells directly inhibit viral replication and subsequent dissemination within a host via the elimination of infected cells. The 2 major means of target-cell cytolysis are cytotoxic granule exocytosis and the Fas/FasL pathway.8 Cytotoxic granules are secretory lysosomes9 that contain multiple proteins—including perforin, granzymes, and granulysin—that work in concert to induce apoptosis in infected cells. The cytotoxic granule pathway is likely the principal mechanism by which HIV-specific CD8+ T cells eliminate HIV-infected cells.6,10 Perforin is a pore-forming protein essential for the entry of various proapoptotic proteases known as granzymes, including Grz A and Grz B, into infected target cells.11-13 Granulysin, a member of the saposin-like protein family, may be important in the control of a wide variety of pathogenic bacteria, fungi, and parasites, and has also been implicated in tumor surveillance.14-16
The transcriptional regulation of cytolytic effector cells has recently become an area of immense interest.17 One transcription factor, T-bet, has been shown to play a pivotal role in the development, differentiation, and function of effector cells. A member of the T-box family,18-20 T-bet (Tbx21) was originally discovered as the major determinant of T-helper 1 (Th1) lineage commitment.21 Subsequent work has demonstrated that it is also important for the effector differentiation of CD8+ T cells.22-24 T-bet is essential for the development of many experimental autoimmune diseases, including type 1 diabetes and myocarditis, in transgenic mice25,26 ; in both models, pathogenic CD8+ T cells are thought to be causally associated with the onset of disease, which is mitigated or prevented in the absence of T-bet. In addition, T-bet can bind to the promoter regions of perforin and Grz B in both human and mouse cells27 and influences the levels of expression of these cytolytic molecules.28,29 Although the biology of T-bet has been well studied in experimental murine models, the possible significance of this transcription factor in the context of human infection and disease is less well understood.
In the present study, we examined the expression of T-bet in both resting and activated CD8+ T cells from HIV-infected and uninfected individuals. We hypothesized that T-bet may play a role in the increased cytotoxic potential that has been described for HIV-specific CD8+ T cells from ECs,5-7 and found that these cells demonstrated higher T-bet expression after both short-term stimulation and in vitro proliferation than cells from individuals who were not controlling HIV replication (“progressors”) or who were controlling virus only through the use of highly active antiretroviral therapy (HAART-suppressed individuals). We also observed that T-bet expression was strongly correlated with perforin and Grz B. Our results suggest that T-bet may influence the effector status of HIV-specific CD8+ T cells and could be an attractive target for modulation to improve HIV-specific immunity.
Methods
Human subjects
We examined HIV-specific CD8+ T-cell responses in a cross-sectional cohort of 20 ECs, 18 chronically infected progressors (CPs), and 19 individuals on HAART. The majority of peripheral blood mononuclear cell (PBMC) specimens were obtained from patients enrolled in the SCOPE study at the University of California-San Francisco.30 PBMC samples from a small number of ECs and CPs were also obtained through clinics associated with the University of Toronto and Harvard University. Blood specimens were acquired with the written informed consent of all study patients in accordance with the Declaration of Helsinki, and with the approval of the institutional review board at each respective institution where patient materials were collected: University of California-San Francisco (IRB# H8211-17 887-10), the University of Toronto and St Michael's Hospital (IRB# 07-106), and Harvard University (IRB# 2003-P-001894 and IRB# 2003-P-001678/75). Clinical information for each subject was numerically coded before being viewed by the investigators who performed the experiments.
ECs were defined as therapy-naive individuals who displayed an initial positive HIV antibody test > 2 years before study entry and who had a minimum of 3 documented viral loads of < 75 copies/mL over a period of 12 months. CPs were defined as untreated individuals with plasma HIV RNA levels consistently above 10 000 copies/mL. HAART-suppressed individuals maintained undetectable plasma viral loads (< 75 copies/mL) for at least a 2-year span before inclusion. All subjects had proximal CD4+ T-cell counts above 350 cells/mm3 and lacked evidence of an AIDS-defining illness (refer to Table 1 for relevant clinical information). In addition, PBMCs from HIV-negative healthy donors were obtained from the University of Pennsylvania Center for AIDS Research Human Immunology Core. PBMCs from all subjects were cryopreserved before use.
HIV-infected cohort clinical information
| Patient characteristics . | ECs (IQR) . | CPs (IQR) . | HAART-suppressed (IQR) . |
|---|---|---|---|
| Number of subjects | 20 | 18 | 19 |
| Median plasma HIV RNA, copies/mL | undetectable | 28 908 (15 904-49 686) | undetectable |
| Median CD4+ T-cell count, cells/mm3 | 772 (664-1133) | 622 (461 734) | 675 (493-868) |
| Median infection duration, y | 17 (11-20) | 6 (3-16) | 14 (10-20) |
| Median duration of HAART treatment prior to specimen sample, y | N/A | N/A | 5 (4-7) |
| Patient characteristics . | ECs (IQR) . | CPs (IQR) . | HAART-suppressed (IQR) . |
|---|---|---|---|
| Number of subjects | 20 | 18 | 19 |
| Median plasma HIV RNA, copies/mL | undetectable | 28 908 (15 904-49 686) | undetectable |
| Median CD4+ T-cell count, cells/mm3 | 772 (664-1133) | 622 (461 734) | 675 (493-868) |
| Median infection duration, y | 17 (11-20) | 6 (3-16) | 14 (10-20) |
| Median duration of HAART treatment prior to specimen sample, y | N/A | N/A | 5 (4-7) |
IQR indicates interquartile range; ECs, elite controllers; CPs, chronically infected progressors; and HAART, highly active antiretroviral therapy.
Antibody reagents
The following antibodies were used in this study: anti-CD8 Qdot 605, anti-CD3 Qdot 655, and anti–Grz B phycoerythrin-Cy5.5 (PE-Cy5.5; Invitrogen); anti–tumor necrosis factor-α (anti-TNFα) PE-Cy7, anti–interferon-γ allophycocyanin (anti–IFN-γ APC), anti-CD4 APC-Cy7, anti-CD14 APC-Cy7, and anti-CD19 APC-Cy7 (BD Pharmingen); anti–granulysin PE, anti-CD107a PE-Cy5, anti-CD27 PE-Cy5, and anti–T-bet PE (clone 4B10; eBioscience); anti–T-bet fluorescein isothiocyanate (anti–T-bet FITC; clone 4B10; Santa Cruz Biotechnology); anti-CD45RO ECD (Beckman Coulter); anti–Grz A Alexa 700 (BioLegend); anti–perforin PE (clone B-D48; Diaclone); and anti-CD57 Qdot 565 and anti–perforin Pacific Blue (custom made). The anti–perforin monoclonal antibody (clone B-D48) purchased from Diaclone was conjugated to Pacific Blue in our laboratory, as were custom conjugations to Qdot nanocrystals with reagents from Invitrogen.
PBMC stimulation and proliferation assays
PBMC stimulations and intracellular cytokine–staining (ICS) assays were carried out as described previously.7 Briefly, PBMCs were resuspended at a concentration of 2 × 106 cells/mL in a total volume of 1 mL for each condition. Costimulatory antibodies (anti-CD28 and anti-CD48d, 1 μg/mL each; BD Biosciences), monensin (1 μg/mL; BD Biosciences), and brefeldin A (1 μg/mL; Sigma-Aldrich) were also added. Anti-CD107a PE-Cy5 was included at the start of all stimulations to measure levels of degranulation.31 PBMCs were incubated at 37°C and 5% CO2 for 6 hours with peptide pools encompassing HIV-1 Gag (PTE), Nef (clade B consensus, NIH AIDS Research and Reference Reagent Program) or human cytomegalovirus (CMV) pp65 and IE1 proteins (New England Peptide). Each individual peptide in the pools was at a final concentration of 2 μg/mL. At the conclusion of the 6-hour stimulation period, cells were labeled with aqua-fluorescent amine-reactive dye (Invitrogen) before staining for surface and intracellular markers, as described previously in detail.32
In some experiments that assessed proliferation, PBMCs were labeled with CFSE (Invitrogen) before stimulation, according to the manufacturer's instructions. Cells were stimulated with individual peptides, the aforementioned HIV Gag or Nef pools, or with a pool of approximately 100 previously identified optimal HIV (clade B) CD8+ T-cell epitopes ranging in length from 9-11 amino acids covering all regions of the viral proteome. Individual peptides were at a final concentration of 2 μg/mL in each condition. At various time points after initial peptide stimulation, antigen-specific cells were identified using peptide: MHC I tetramer (conjugated to APC) or after peptide restimulation for 6 hours in the presence of brefeldin A.
Flow cytometric analysis
For each stimulation condition, at least 500 000 total events were acquired using a modified LSRII (BD Immunocytometry Systems). Data analysis was performed using FlowJo (Version 8.8.4; TreeStar) and Spice (Version 4.2.3; Dr Mario Roederer, NIH, Bethesda, MD) software. The gating strategy was similar to that used in our previous work.7 Briefly, we gated on singlets, dump− cells, viable cells (Aqua Blue−), lymphocytes, CD3+ cells, and CD8+ cells before finally gating on functional cells. Reported data have been corrected for background based on the negative (no peptide) control where appropriate, and only responses with a total frequency above 0.10% of total CD8+ T cells (after background subtraction) were considered to be positive responses. The contribution of perforin, Grz A, Grz B, or granulysin to all HIV- or CMV-specific CD8+ T-cell responses was calculated individually in combination with IFN-γ+, TNFα+, or CD107a+ cells. Therefore, in each instance, a total of 16 possible functional combinations was possible. As in our previous study,7 2 combinations were ignored in subsequent analysis: (1) events negative for all parameters (ie, IFN-γ−TNFα−CD107a−cytolytic protein−) and (2) cytolytic molecule single-positive cells (ie, perforin single-positive cells, Grz A single-positive cells, etc). By analyzing the data in this manner, we examined cytolytic protein production resulting from antigen-specific stimulation and ensured that its expression was considered only within activated CD8+ T cells expressing at least one other functional parameter. Whereas IFN-γ, TNFα, and surface expression of CD107a were used to identify antigen-specific CD8+ T cells, only responses producing IFN-γ are depicted in all figures for the sake of simplicity and for ease of data presentation.
Statistical analysis
All statistical analysis was performed using GraphPad Prism software (Version 5.0a). Functionality was compared between study groups using nonparametric tests: Mann-Whitney for comparing only 2 groups and Kruskal-Wallis followed by a Dunn test for multiple comparisons comparing 3 or more groups. Correlations were assessed using the Spearman test.
Results
Expression pattern of cytotoxic, granule-resident proteins in bulk CD8+ T cells
We recently reported that HIV-specific CD8+ T cells from ECs demonstrated an enhanced ability to express perforin directly ex vivo compared with CPs.7 By measuring a more comprehensive array of cytolytic proteins in this work, we sought a deeper understanding of CD8+ T-cell–cytotoxic potential among individuals who demonstrate differential control over HIV replication. We studied CD8+ T-cell responses using a flow cytometric staining panel that was designed to simultaneously measure the expression of Grz A, Grz B, granulysin, and perforin (representative staining of each shown in supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We used an anti–perforin antibody that can detect both preformed and newly up-regulated perforin after activation.7,32,33
Initially, we characterized the coexpression of these 4 molecules in bulk CD8+ T cells among HIV-negative donors before moving into HIV-infected individuals (n = 4; supplemental Figure 1B). In general, Grz A was the most ubiquitously expressed molecule, being present in virtually every combination of cytolytic molecules, which is in agreement with previous findings.34,35 Approximately 25% of the total CD8+ T-cell compartment coexpressed all 4 molecules, whereas, on average, 50% of all CD8+ T cells expressed either none of the cytolytic proteins or Grz A alone. The majority of granulysin was coexpressed with Grz A, Grz B, and perforin; however, a sizeable fraction of granulysin was observed with either Grz A or Grz B but not perforin. Therefore, our staining panel appeared valid and produced results consistent with prior studies.34,36 On examination of the HIV-positive cohort, the overall expression patterns of Grz A, Grz B, granulysin, and perforin in the total CD8+ T-cell compartment was found to be similar (data not shown). We also found no differences among ECs, CPs, and HAART-suppressed patients in the proportion of the total CD8+ T-cell pool that expressed Grz A, Grz B, granulysin, or perforin as single parameters (supplemental Figure 1C).
HIV-specific CD8+ T cells in ECs demonstrated a greater ability to express perforin and Grz B compared with CPs and HAART-suppressed subjects
We next identified HIV- and CMV-specific CD8+ T-cell responses among subjects in the HIV-infected cohort by measuring levels of IFN-γ, TNFα, and degranulation after stimulation with overlapping peptide pools. As shown in supplemental Figure 2, the total CD8+ T-cell response magnitude to HIV- or CMV-specific stimulation was similar between the groups, which is consistent with previous studies.5,7,30,37 As expected,38 the total CMV-specific response was greater than the HIV-specific response across all groups.
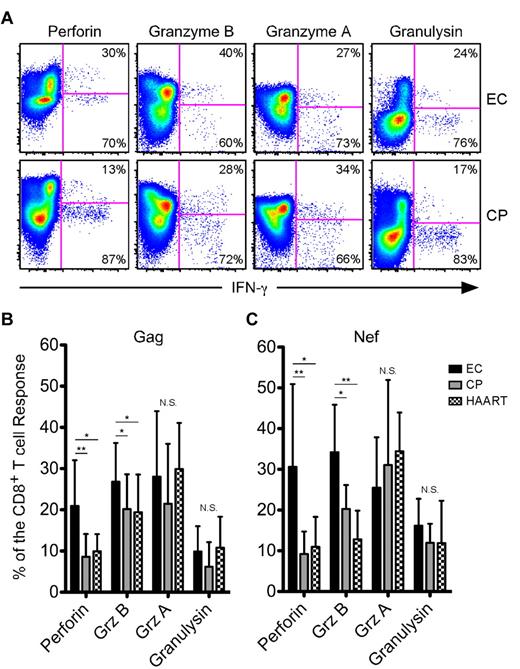
We subsequently determined the contribution of Grz A, Grz B, granulysin, and perforin to the HIV-specific CD8+ T-cell response in each subject. We consistently observed higher expression of perforin and Grz B within responding cells from ECs compared with CPs or HAART-suppressed individuals (Figure 1A shows example Nef-specific responses; a HAART-suppressed example is not shown); however, coexpression of Grz A and granulysin within responding cells appeared similar across the cohort (Figure 1A). On examination of the entire HIV-infected cohort in a quantitative manner, perforin and Grz B were found to comprise a significantly greater proportion of the average Gag-specific (Figure 1B) and Nef-specific (Figure 1C) responses in ECs compared with both CPs and HAART-suppressed subjects, whereas the proportion of the HIV-specific CD8+ T-cell responses composed of Grz A or granulysin did not differ between the groups (Figure 1B-C).
HIV-specific CD8+ T cells from ECs express higher amounts of perforin and Grz B than CPs after short-term stimulation. (A) Representative flow cytometric plots showing perforin, Grz B, Grz A, and granulysin versus IFN-γ from ECs (top row) and CPs (bottom row). Percentages represent the proportion of IFN-γ–expressing cells that were either positive or negative for each cytolytic protein. The determination of gate placement was aided by consideration of cytolytic protein expression, which is often low or absent, within CD19+ B cells or CD4+ T cells. (B-C) Relative contribution of perforin, Grz B, Grz A, and granulysin to Gag-specific (B) and Nef-specific (C) CD8+ T-cell responses, as identified by staining for IFN-γ, TNFα, or CD107a, is shown among all ECs, CPs, and HAART subjects. For each cytolytic molecule, statistical analysis was carried out using 1-way ANOVA tests (nonparametric; Kruskal-Wallis) followed by a Dunn test for multiple comparisons. *P < .05; **P < .01. Bars represent the means and error bars indicate SDs.
HIV-specific CD8+ T cells from ECs express higher amounts of perforin and Grz B than CPs after short-term stimulation. (A) Representative flow cytometric plots showing perforin, Grz B, Grz A, and granulysin versus IFN-γ from ECs (top row) and CPs (bottom row). Percentages represent the proportion of IFN-γ–expressing cells that were either positive or negative for each cytolytic protein. The determination of gate placement was aided by consideration of cytolytic protein expression, which is often low or absent, within CD19+ B cells or CD4+ T cells. (B-C) Relative contribution of perforin, Grz B, Grz A, and granulysin to Gag-specific (B) and Nef-specific (C) CD8+ T-cell responses, as identified by staining for IFN-γ, TNFα, or CD107a, is shown among all ECs, CPs, and HAART subjects. For each cytolytic molecule, statistical analysis was carried out using 1-way ANOVA tests (nonparametric; Kruskal-Wallis) followed by a Dunn test for multiple comparisons. *P < .05; **P < .01. Bars represent the means and error bars indicate SDs.
We also examined the levels of Grz A, Grz B, granulysin, and perforin in CMV-specific responses in each group. We found no difference in the proportion of the CMV-specific response composed of any measured cytolytic molecule between ECs and CPs. However, HAART-suppressed subjects demonstrated lower levels of perforin and Grz B than ECs or CPs, but with no difference in the proportion of Grz A or granulysin (supplemental Figure 3). In summary, HIV-specific CD8+ T cells from ECs produced higher levels of perforin and Grz B directly ex vivo compared with either CPs or HAART subjects, but we found no difference in the levels of Grz A or granulysin between any cohort group after either HIV- or CMV-specific stimulation. Because of the lack of difference in the levels of Grz A or granulysin between the cohort groups, we largely focused on perforin and Grz B for the remainder of the study.
T-bet expression was highly associated with effector phenotype and cytotoxic potential in resting CD8+ T cells
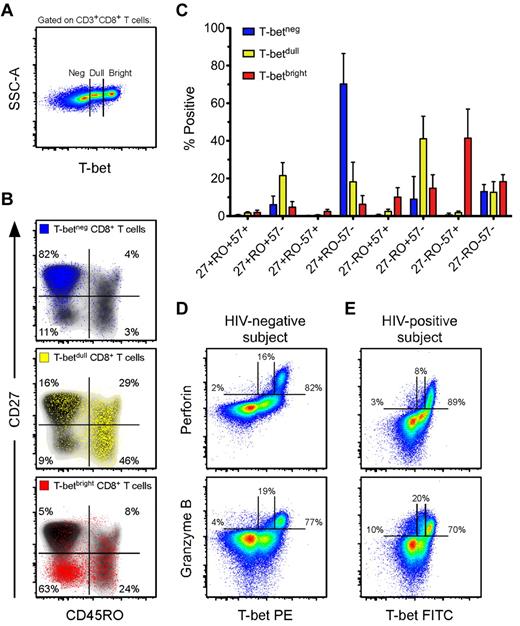
Given that the T-box transcription factor T-bet has been shown to influence effector CD8+ T-cell differentiation in mice,23 and that previous work from our laboratory has associated T-bet with effector CD8+ T cells in humans,39 we hypothesized that T-bet may be associated with the effector differentiation and cytotoxic potential of HIV-specific CD8+ T cells within ECs. Initially, we characterized the memory phenotype and cytotoxic potential of human CD8+ T cells that express T-bet, which had not been previously examined in detail. We determined the expression patterns of T-bet among bulk CD8+ T cells from a group of HIV-negative donors (n = 8). As shown in Figure 2A, we consistently observed 3 populations of T-bet: T-betneg, T-betdull, and T-betbright. The T-betneg cells were almost exclusively CD27+CD45RO−, the majority of T-betdull cells were CD45RO+ with variable expression of CD27, and the T-betbright cells were primarily CD27−CD45RO− (Figure 2B). Therefore, the majority of T-betbright cells displayed the typical phenotypic characteristics of differentiated effector cells.40
Effector CD8+ T cells contain the highest levels of T-bet, which is associated with perforin and Grz B expression in resting cells. (A) Three populations of T-bet are typically observed in human CD8+ T cells. Shown is a representative flow cytometric plot of the T-bet staining profile within CD3+CD8+ T cells from an HIV-negative subject. (B) Representative flow cytometric plots of the memory distribution, as determined by CD27 and CD45RO, of the 3 populations of T-bet: negative (blue), dull (yellow), and bright (red). These various T-bet populations were overlaid onto density plots (black shading) of total CD8+ T cells. Percentages represent the fraction of overlaid cells that fell within each quadrant. (C) The memory distributions of T-betneg (blue), T-betdull (yellow), and T-betbright (red) populations, as determined by CD27, CD45RO, and CD57, were established for HIV-negative subjects (n = 8). Bars represent the means and error bars indicate SDs. (D-E) Representative flow cytometric plots showing T-bet against both perforin and Grz B among total CD8+ T cells from an HIV-negative (D) and an HIV-positive (E) subject. Percentages represent the fraction of perforin or Grz B that was negative, dull, or bright for T-bet expression.
Effector CD8+ T cells contain the highest levels of T-bet, which is associated with perforin and Grz B expression in resting cells. (A) Three populations of T-bet are typically observed in human CD8+ T cells. Shown is a representative flow cytometric plot of the T-bet staining profile within CD3+CD8+ T cells from an HIV-negative subject. (B) Representative flow cytometric plots of the memory distribution, as determined by CD27 and CD45RO, of the 3 populations of T-bet: negative (blue), dull (yellow), and bright (red). These various T-bet populations were overlaid onto density plots (black shading) of total CD8+ T cells. Percentages represent the fraction of overlaid cells that fell within each quadrant. (C) The memory distributions of T-betneg (blue), T-betdull (yellow), and T-betbright (red) populations, as determined by CD27, CD45RO, and CD57, were established for HIV-negative subjects (n = 8). Bars represent the means and error bars indicate SDs. (D-E) Representative flow cytometric plots showing T-bet against both perforin and Grz B among total CD8+ T cells from an HIV-negative (D) and an HIV-positive (E) subject. Percentages represent the fraction of perforin or Grz B that was negative, dull, or bright for T-bet expression.
CD57 is a memory marker that is commonly associated with an effector phenotype.36,41 CD8+ T cells that lacked or expressed intermediate levels of T-bet were virtually all CD57− (Figure 2C). The majority of T-betbright cells expressed CD57; however, a sizeable fraction were CD27−CD45RO+/−CD57− (Figure 2C). Thus, CD57 was associated with the highest levels of T-bet expression, but T-betbright cells were not exclusively CD57+. These data suggest that most T-betbright CD8+ T cells are differentiated effector cells. Cells of this memory phenotype have been shown previously to express high levels of perforin and Grz B.34,36 Consequently, T-betbright CD8+ T cells expressed perforin and Grz B most abundantly compared with both the T-betneg or T-betdull populations (representative example shown in Figure 2D). These associations between T-bet and cytolytic protein expression were also maintained in the HIV-infected cohort (representative example shown in Figure 2E); indeed, we found a highly significant positive correlation between T-betbright levels and the expression of both perforin and Grz B in the total CD8+ T-cell pool among the entire HIV-positive cohort (supplemental Figure 4).
HIV-specific CD8+ T cells from ECs expressed higher levels of T-bet than CPs or HAART-suppressed subjects
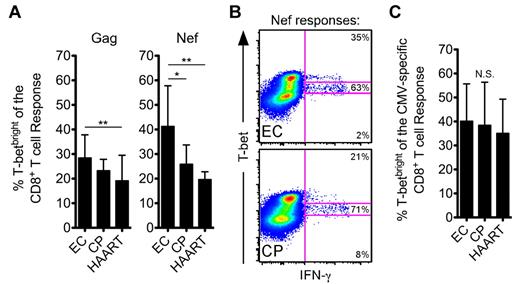
We next examined T-bet expression within the HIV-positive cohorts. A significantly higher fraction of HIV-specific CD8+ T-cell responses were T-betbright in ECs than in CPs and/or HAART-suppressed individuals (Figure 3A), because a larger percentage of responding HIV-specific CD8+ T cells among ECs fell within the T-betbright gate than among CPs (example Nef responses are shown in Figure 3B). However, there was no difference between the groups in T-bet expression among bulk CD8+ T cells (supplemental Figure 5). Moreover, T-bet levels did not differ for CMV-specific CD8+ T-cell responses between ECs, CPs, and HAART subjects (Figure 3C), which suggests that CPs do not suffer from a global immune defect.
HIV-specific CD8+ T cells from ECs express higher amounts of T-bet than CPs after short-term stimulation. (A) The fraction of Gag- or Nef-specific CD8+ T cells, as identified by staining for IFN-γ, TNFα, or CD107a, that fell within the T-betbright gate was determined for all ECs, CPs, and HAART subjects. (B) Representative flow cytometric plots from ECs and CPs showing the fraction of the Nef-specific response that fell within the 3 T-bet gates. Events shown have been gated on CD8+ T cells. (C) The fraction of CMV-specific CD8+ T cells that fell within the T-betbright gate was determined for all ECs, CPs, and HAART subjects as in panel A. (A-C) Statistical analysis was carried out using 1-way ANOVA (nonparametric; Kruskal-Wallis) followed by a Dunn test for multiple comparisons. *P < .05; **P < .01. Bars represent the means and error bars indicate SDs.
HIV-specific CD8+ T cells from ECs express higher amounts of T-bet than CPs after short-term stimulation. (A) The fraction of Gag- or Nef-specific CD8+ T cells, as identified by staining for IFN-γ, TNFα, or CD107a, that fell within the T-betbright gate was determined for all ECs, CPs, and HAART subjects. (B) Representative flow cytometric plots from ECs and CPs showing the fraction of the Nef-specific response that fell within the 3 T-bet gates. Events shown have been gated on CD8+ T cells. (C) The fraction of CMV-specific CD8+ T cells that fell within the T-betbright gate was determined for all ECs, CPs, and HAART subjects as in panel A. (A-C) Statistical analysis was carried out using 1-way ANOVA (nonparametric; Kruskal-Wallis) followed by a Dunn test for multiple comparisons. *P < .05; **P < .01. Bars represent the means and error bars indicate SDs.
T-bet was highly associated with perforin and Grz B expression in antigen-specific CD8+ T cells after short-term stimulation
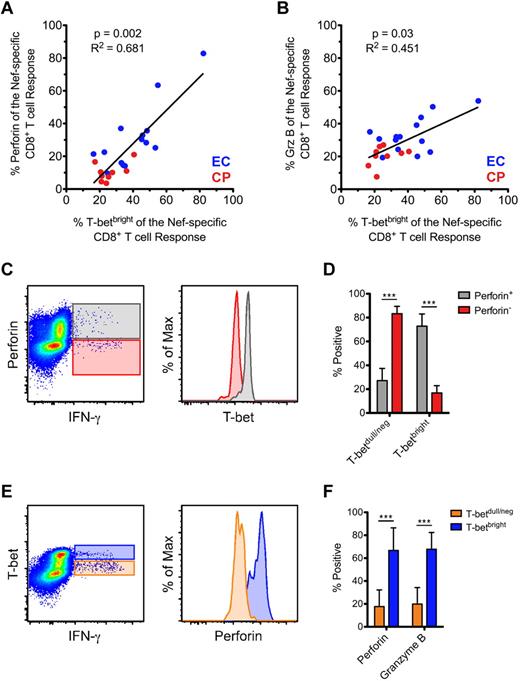
We found a positive correlation between the levels of Nef-specific perforin (Figure 4A) and Grz B (Figure 4B) and the fraction of the response that was T-betbright; however, we found no correlation between levels of Nef-specific Grz A or granulysin and the fraction of the response that was T-betbright (data not shown). Similar overall trends were also observed for CMV- and Gag-specific responses (data not shown). Therefore, the correlation between T-bet and perforin and Grz B that we observed within resting cells (Figure 2) also appeared to be maintained among stimulated CD8+ T cells.
Positive correlation between T-bet and the expression of both perforin and Grz B within activated CD8+ T cells. (A-B) The proportion of Nef-specific perforin (A) and Grz B (B) expression was plotted against the fraction of the response that was T-betbright within all therapy-naive subjects (ECs, blue; CPs, red). Spearman correlation tests (nonparametric; 2-tailed) were performed to determine statistical significance as indicated. Only those subjects in whom a Nef-specific response was detected are plotted. (C) Relative levels of T-bet expression are shown within perforin+IFN-γ+ (gray) and perforin−IFN-γ+ (red) CD8+ T cells from an example Nef-specific response. (D) T-bet expression profile of all perforin+ and perforin− Nef-specific CD8+ T cells (as identified by staining for IFN-γ, TNFα, or CD107a) was determined among ECs. (E) Relative levels of perforin expression are shown within T-betbrightIFN-γ+ (blue) and T-betdull/negIFN-γ+ (orange) CD8+ T cells from an example Nef-specific response. (F) The degree of perforin and Grz B positivity was determined for all T-betbright and T-betdull/neg Nef-specific CD8+ T cells (as identified by staining for IFN-γ, TNFα, or CD107a) among ECs. (D and F) Mann-Whitney tests (nonparametric; 2-tailed) were performed to determine statistical significance. ***P < .001. Bars represent the means and error bars indicate SDs.
Positive correlation between T-bet and the expression of both perforin and Grz B within activated CD8+ T cells. (A-B) The proportion of Nef-specific perforin (A) and Grz B (B) expression was plotted against the fraction of the response that was T-betbright within all therapy-naive subjects (ECs, blue; CPs, red). Spearman correlation tests (nonparametric; 2-tailed) were performed to determine statistical significance as indicated. Only those subjects in whom a Nef-specific response was detected are plotted. (C) Relative levels of T-bet expression are shown within perforin+IFN-γ+ (gray) and perforin−IFN-γ+ (red) CD8+ T cells from an example Nef-specific response. (D) T-bet expression profile of all perforin+ and perforin− Nef-specific CD8+ T cells (as identified by staining for IFN-γ, TNFα, or CD107a) was determined among ECs. (E) Relative levels of perforin expression are shown within T-betbrightIFN-γ+ (blue) and T-betdull/negIFN-γ+ (orange) CD8+ T cells from an example Nef-specific response. (F) The degree of perforin and Grz B positivity was determined for all T-betbright and T-betdull/neg Nef-specific CD8+ T cells (as identified by staining for IFN-γ, TNFα, or CD107a) among ECs. (D and F) Mann-Whitney tests (nonparametric; 2-tailed) were performed to determine statistical significance. ***P < .001. Bars represent the means and error bars indicate SDs.
We examined in detail the relationship between T-bet, perforin, and Grz B within short-term–stimulated CD8+ T cells. Among HIV-specific CD8+ T cells, perforin+ responding cells consistently contained higher levels of T-bet than perforin− cells (representative example shown in Figure 4C for a Nef-specific response). Upon quantification of all Nef-specific CD8+ T cells among ECs, perforin+ cells expressed significantly higher levels of T-bet than perforin− cells (Figure 4D); Grz B+ Nef-specific CD8+ T cells also expressed significantly higher levels of T-bet than Grz B− cells (data not shown). We subsequently examined the same responses in a reciprocal fashion. Among HIV-specific CD8+ T cells, T-betbright responding cells contained higher levels of perforin and Grz B than T-betdull/neg cells (representative example shown in Figure 4E for a Nef-specific response; Grz B data not shown). Upon quantification of all Nef-specific CD8+ T cells among ECs, T-betbright cells expressed significantly higher levels of both perforin and Grz B than T-betdull/neg cells (Figure 4F). Similar trends were observed in CPs and for Gag-specific responses (data not shown).
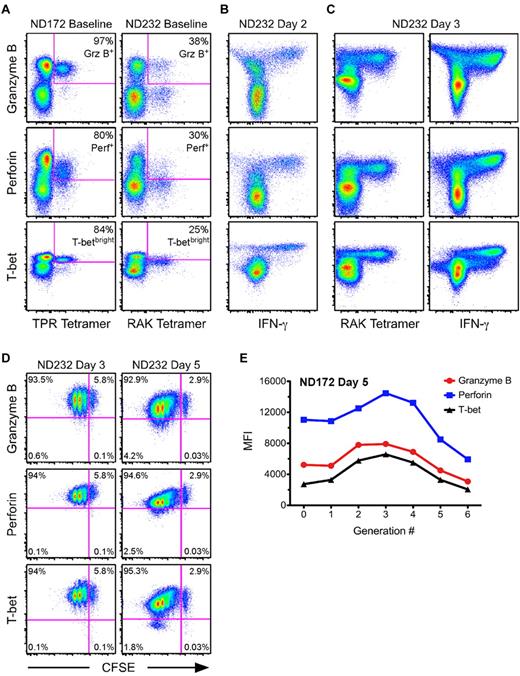
T-bet was rapidly up-regulated along with perforin and Grz B during virus-specific recall responses
To determine how the expression of T-bet was modulated over the course of an antigen-specific recall response, we began by examining 2 HIV-negative donors in whom we had previously identified high-magnitude, peptide-specific CD8+ T-cell responses. ND172 had a CD8+ T-cell response directed against the HLA-B7–restricted epitope TPRVTGGGAM from the human CMV pp65 tegument protein. Approximately 10% of all CD8+ T cells were TPR specific as shown by tetramer staining directly ex vivo in this donor (data not shown). The second subject, ND232, had a CD8+ T-cell response to the HLA-B8–restricted epitope RAKFKQLL derived from the EBV BZLF1 protein. Approximately 5% of total CD8+ T cells were RAK specific as shown by tetramer staining at baseline (data not shown).
Before in vitro stimulation, a much higher fraction of the TPR-specific cells compared with the RAK-specific cells were positive for perforin (80% vs 30%) and Grz B (97% vs 38%; Figure 5A). In addition, 84% of the TPR-specific cells were T-betbright at baseline, whereas only 25% of RAK-specific cells achieved a similar level of T-bet expression (Figure 5A). Of the T-betdull RAK-specific cells, only 9% were perforin+Grz B+, whereas 72% of T-betbright RAK-specific cells were positive for both cytolytic proteins (supplemental Figure 6). We subsequently performed in vitro proliferation assays using CFSE-labeled PBMCs, and examined levels of T-bet, perforin, and Grz B within antigen-specific cells. After 24 hours, there was complete loss of the ability to identify TPR- or RAK-specific CD8+ T cells using tetramer (data not shown). Thus, to identify antigen-specific cells, we performed overnight IFN-γ ICS assays using TPR or RAK peptides. There were no substantial changes in T-bet, perforin, or Grz B levels at this time point compared with the expression profiles at baseline (data not shown). This result was confirmed in 5 additional HIV-negative subjects by comparing T-bet expression levels at 6 and 24 hours after staphylococcal enterotoxin B stimulation (supplemental Figure 7).
Kinetics of T-bet, perforin, and Grz B up-regulation are tightly linked during an antigen-specific recall response. (A) The degree of Grz B, perforin, and T-betbright positivity was determined at baseline for TPR- and RAK-specific CD8+ T cells (labeled by the peptide: MHC I tetramer) from 2 HIV-negative donors, ND172 and ND232, respectively. Percentages represent the fraction of tetramer+ cells that fell within each gate. (B-C) PBMCs from ND232 were incubated for either 2 (B) or 3 (C) days in the presence of RAK peptide. The levels of Grz B, perforin, and T-bet are shown within RAK-specific cells, which were identified either by peptide restimulation for 6 hours or by tetramer staining. Events shown have been gated on CD8+ T cells. (D) CFSE-labeled PBMCs from ND232 were incubated for either 3 or 5 days in the presence of RAK peptide, and the expression of Grz B, perforin, and T-bet was subsequently determined. All flow cytometric plots show only RAK-specific cells, as determined by tetramer staining. Percentages represent the fraction of tetramer+ cells that fell within each quadrant. (E) CFSE-labeled PBMCs from ND172 were incubated for 5 days in the presence of the TPR peptide. The MFI of Grz B, perforin, and T-bet within TPR-specific cells was plotted against each generation number, which was established with FlowJo software Version 8.8.4.
Kinetics of T-bet, perforin, and Grz B up-regulation are tightly linked during an antigen-specific recall response. (A) The degree of Grz B, perforin, and T-betbright positivity was determined at baseline for TPR- and RAK-specific CD8+ T cells (labeled by the peptide: MHC I tetramer) from 2 HIV-negative donors, ND172 and ND232, respectively. Percentages represent the fraction of tetramer+ cells that fell within each gate. (B-C) PBMCs from ND232 were incubated for either 2 (B) or 3 (C) days in the presence of RAK peptide. The levels of Grz B, perforin, and T-bet are shown within RAK-specific cells, which were identified either by peptide restimulation for 6 hours or by tetramer staining. Events shown have been gated on CD8+ T cells. (D) CFSE-labeled PBMCs from ND232 were incubated for either 3 or 5 days in the presence of RAK peptide, and the expression of Grz B, perforin, and T-bet was subsequently determined. All flow cytometric plots show only RAK-specific cells, as determined by tetramer staining. Percentages represent the fraction of tetramer+ cells that fell within each quadrant. (E) CFSE-labeled PBMCs from ND172 were incubated for 5 days in the presence of the TPR peptide. The MFI of Grz B, perforin, and T-bet within TPR-specific cells was plotted against each generation number, which was established with FlowJo software Version 8.8.4.
We observed a dramatic change in the expression of T-bet, perforin, and Grz B within RAK-specific cells at day 2 (Figure 5B). Indeed, by 48 hours after initial activation, all detectable RAK-specific cells were now positive for T-bet, perforin, and Grz B; a similar phenomenon was observed for TPR-specific cells (data not shown). Interestingly, the median fluorescence intensity (MFI) of T-bet within RAK-specific cells at day 2 was higher than the MFI of the T-betbright population at baseline in ND232 (compare Figure 5B with 5A). In fact, an apparent fourth population of T-bet–expressing cells (T-bet+++) appeared by 48 hours (Figure 5B); this phenomenon was not observed in the negative (DMSO/no peptide) control (data not shown). The ability to stain RAK-specific cells with tetramer returned by day 3 (Figure 5C). Consistent with the 48-hour time point, all RAK tetramer+ cells were Grz B+, perforin+, and T-bet+++ by 72 hours; peptide restimulation at day 3 revealed similar results (Figure 5C). The appearance of a T-bet+++ population also occurred in ND172 (data not shown).
We next examined the relationship between cell division and the modulation of T-bet, perforin, and Grz B. The MFI of these proteins within antigen-specific cells initially increased before decreasing slightly with each subsequent round of cell division (RAK-specific cells are shown in Figure 5D). Upon quantification of the MFI of T-bet, perforin, and Grz B within each generation of tetramer+ cells at day 5, we observed that the kinetics of up-regulation were tightly correlated for both TPR-specific cells in ND172 (Figure 5E) and RAK-specific cells in ND232 (data not shown). Thus, the proliferation of peptide-specific CD8+ T cells during a recall response was associated with concomitant up-regulation of T-bet, perforin, and Grz B.
HIV-specific CD8+ T cells from ECs displayed higher T-bet expression than CPs after proliferation
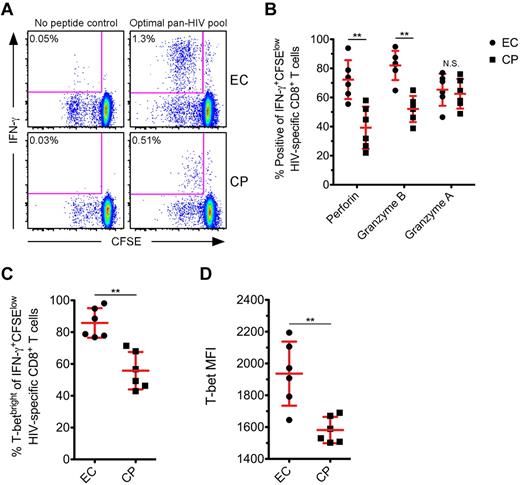
Finally, we studied the levels of T-bet, perforin, Grz B, and Grz A after proliferation of HIV-specific CD8+ T cells. We compared the expression of these proteins in a subset of ECs and CPs after 5 days of in vitro stimulation with various HIV peptide pools. As expected,5,42 HIV-specific CD8+ T cells from ECs demonstrated a greater proliferative ability than CPs (representative examples shown in Figure 6A). A median of 1.01% of CD8+ T cells diluted CFSE after HIV-specific stimulation in ECs, whereas only a median of 0.41% of CD8+ T cells did so in CPs (P = .03; data not shown). A significantly higher fraction of IFN-γ+CFSElow cells from ECs expressed perforin and Grz B compared with CPs at day 5 (Figure 6B); however, there was no difference in the up-regulation of Grz A between the 2 groups (Figure 6B), which is in agreement with a prior report.6 There were significantly higher levels of T-bet within IFN-γ+CFSElow cells from ECs compared with CPs (Figure 6C-D), and we found a positive correlation between the levels of T-bet within IFN-γ+CFSElow cells and the corresponding amount of perforin and Grz B expression for each respective subject (data not shown).
HIV-specific CD8+ T cells from ECs express higher amounts of T-bet than CPs after proliferation. (A) CFSE-labeled PMBCs from a subset of ECs and CPs were stimulated for 5 days in the presence of a pool of Nef, Gag, or optimal 9-11mer HIV peptides. Representative flow cytometric plots show HIV-specific CD8+ T cells, as determined by the production of IFN-γ after restimulation, that have proliferated. Percentages represent the fraction of total CD8+ T cells within each gate. (B) The proportion of IFN-γ+CFSElow HIV-specific CD8+ T cells positive for perforin, Grz B, and Grz A was determined on day 5 for ECs and CPs. (C-D) T-bet expression was also determined on day 5 after HIV-specific stimulation, with levels reported as the percentage (C) or MFI (D) among IFN-γ+CFSElow HIV-specific CD8+ T cells. The reported MFI values indicate the difference between the T-betneg population and the T-bet MFI of the IFN-γ+CFSElow cells for each subject. (B-D) Mann-Whitney tests (nonparametric; 2-tailed) were performed to compare ECs with CPs for each functional parameter. **P < .01. Error bars indicate the means and SDs.
HIV-specific CD8+ T cells from ECs express higher amounts of T-bet than CPs after proliferation. (A) CFSE-labeled PMBCs from a subset of ECs and CPs were stimulated for 5 days in the presence of a pool of Nef, Gag, or optimal 9-11mer HIV peptides. Representative flow cytometric plots show HIV-specific CD8+ T cells, as determined by the production of IFN-γ after restimulation, that have proliferated. Percentages represent the fraction of total CD8+ T cells within each gate. (B) The proportion of IFN-γ+CFSElow HIV-specific CD8+ T cells positive for perforin, Grz B, and Grz A was determined on day 5 for ECs and CPs. (C-D) T-bet expression was also determined on day 5 after HIV-specific stimulation, with levels reported as the percentage (C) or MFI (D) among IFN-γ+CFSElow HIV-specific CD8+ T cells. The reported MFI values indicate the difference between the T-betneg population and the T-bet MFI of the IFN-γ+CFSElow cells for each subject. (B-D) Mann-Whitney tests (nonparametric; 2-tailed) were performed to compare ECs with CPs for each functional parameter. **P < .01. Error bars indicate the means and SDs.
Discussion
Several lines of evidence have suggested that the presence and maintenance of effector function by HIV-specific CD8+ T cells is critical for the control of viral replication. In this study, we comprehensively examined the expression of various cytolytic proteins by HIV-specific CD8+ T cells. We found that HIV-specific CD8+ T cells from ECs demonstrated higher perforin and Grz B expression than HIV-infected CPs or HAART-suppressed individuals; however, we found no difference in the levels of Grz A or granulysin between the groups. We observed that T-bet, a critical transcriptional regulator of effector CD8+ T-cell differentiation and function,22-24 was expressed to a higher degree in HIV-specific CD8+ T cells from ECs compared with CPs. Furthermore, the link between T-bet and perforin and Grz B was observed after short-term activation and upon proliferation of antigen-specific CD8+ T cells. Because of limited cell availability, we assessed HIV-specific responses by stimulating with Gag and Nef peptide pools. However, based on our prior work,7 it seems likely that the findings reported in this study would extend to other HIV antigens as well.
We observed higher expression of perforin and Grz B from HIV-specific CD8+ T cells in ECs compared with CPs using ex vivo ICS assays. It is known that there is a tendency for perforin-expressing CD8+ T cells to coexpress Grz B both at rest34 and after activation.39 In agreement with this, we found a positive correlation between the expression of perforin and Grz B within activated CD8+ T cells (data not shown). Whereas we did not perform in vitro killing assays (mainly because of the technical constraints associated with ex vivo cells), our data suggest that HIV-specific CD8+ T cells in ECs have a superior cytotoxic potential by expressing higher levels of perforin and Grz B. Indeed, recent work from Migueles et al6 demonstrated that higher amounts of perforin and Grz B within in vitro–expanded CD8+ T cells translated into an enhanced ability to lyse infected targets on a per-cell basis. Therefore, our findings suggest that HIV-specific CD8+ T cells in ECs have the potential to eliminate infected targets to a greater degree than those in CPs. Whereas HIV-specific CD8+ T cells from both ECs and CPs express ample amounts of Grz A, ECs generally contain higher amounts of perforin and Grz B relative to CPs. Prior research has implicated Grz B as the major effector granzyme that induces target-cell death, whereas Grz A has been shown to be less cytotoxic or not at all.12 Accordingly, Harari et al reported a positive correlation between target-cell lysis and the levels of perforin and/or Grz B in virus-specific CD8+ T cells35 ; however, no such relationship was found between levels of Grz A and target-cell destruction in the same report. Therefore, HIV-specific CD8+ T cells from ECs in general contain higher amounts of the cytotoxic molecules previously shown to be crucial to target-cell cytolysis, which most likely explains the increased cytotoxic capabilities of HIV-specific CD8+ T cells from ECs.3,4,6,43 The fact that there was no difference in the production of perforin or Grz B by CMV-specific CD8+ T cells between ECs and CPs implies an HIV-specific phenomenon rather than a global deficiency in effector function within CPs. In addition, our results indicate that low HIV viral load by itself (through the use of HAART) is unlikely to be an explanation for the higher levels of perforin and Grz B seen in ECs, a finding that is consistent with previous reports.7,44
We recently reported that there is an enrichment of HIV-specific effector CD8+ T cells in ECs that express perforin.7 The memory phenotype of these perforin-expressing cells was primarily CD27−CD45RO−CD57+/−. The fact that T-betbright cells displayed a strikingly similar memory phenotype, combined with the positive correlation with perforin, led us to hypothesize that there may be higher levels of T-bet within HIV-specific CD8+ T cells from ECs compared with CPs. Whereas it was originally discovered as the major determinant of Th1 lineage commitment,21 T-bet has subsequently been shown to be important for the differentiation and function of effector CD8+ T cells as well.22-24 In this study, we identified 3 distinct populations of T-bet within human CD8+ T cells: a bright, a dull, and a negative population. Interestingly, we found that the T-betbright population expressed the highest levels of perforin and Grz B, which is indicative of elevated cytotoxic potential within human antigen–specific CD8+ T cells.6
The association we found between T-bet, perforin, and Grz B in resting or short-term activated CD8+ T cells was accentuated after in vitro proliferation. For example, EBV-specific CD8+ T cells from one of our HIV-negative donors demonstrated a dramatic capacity to simultaneously up-regulate T-bet, perforin, and Grz B within 2 days of in vitro activation despite initially expressing low levels of these proteins. We also observed that dividing HIV-specific CD8+ T cells from ECs displayed a greater ability than CPs to up-regulate T-bet, perforin, and Grz B after in vitro proliferation, an extension of the prior work performed by Migueles et al.5,6 Further work needs to be done to manipulate T-bet expression (knock-down or overexpression) to formally show a causative role for T-bet in promoting the expression of perforin and/or Grz B. Nevertheless, our data do show a very tight association between T-bet and the presence of these cytolytic molecules. Furthermore, a recent study that tracked CMV-seronegative recipients of seropositive donor kidneys showed a correlation between T-bet and effector status in the setting of human CMV–specific CD8+ T cells.45
No studies of HIV-infected individuals have shown polymorphisms in granule-resident proteins to be associated with virologic control or progression status. Therefore, all available data suggest that perforin and Grz B molecules themselves are equally functional between ECs and CPs. Our work suggests that the underlying defect(s) in effector function by HIV-specific CD8+ T cells from CPs lies not in the cytolytic molecules themselves, but rather in the elements controlling their expression. This study reinforces the importance of understanding the regulatory elements that influence effector function, which is an area of increasing research attention.17,28,29,46-49 Other transcription factors in addition to or in combination with T-bet may promote effector function in various settings. For example, several studies have reported that eomesodermin, a T-box family member with T-bet, may be important to effector differentiation in the murine system.28,29 Whereas we focused solely on T-bet for this study, future work should address the potential contribution of other regulatory factors to the expression of cytolytic molecules in the context of HIV infection or other human pathogens. Our results point to a link between T-bet and both effector status and the expression of perforin and Grz B with human CD8+ T cells. Therapeutic strategies aimed at modulating T-bet expression may lead to enhanced effector activity against HIV with the goal of enhanced virologic control and delayed disease progression among infected individuals.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Jay Gardner for performing antibody conjugations in our laboratory and the study participants for donating material for this study.
This work was supported by many sources, including the National Institutes of Health (grants RO1 AI076066 and HHSN272200800063C to M.B.), the Centers for AIDS Research at the University of California-San Francisco (grant PO AI27763), the Center for AIDS Research Network of Integrated Systems (grant R24 AI067039), the University of California-San Francisco Clinical and Translational Science Institute (grant UL1 RR024131), the National Institute of Allergy and Infectious Diseases (grants RO1 AI087145, K24AI069994, and AI 76174), the American Foundation for AIDS Research, and the Ragon Institute.
National Institutes of Health
Authorship
Contribution: A.R.H. and M.R.B. conceived of and designed the experiments and wrote the paper; A.R.H. and G.L.C. performed the experiments; A.R.H. analyzed the data; and J.N.M., L.Y.S., P.M.S., C.M.K., G.M., R.K., F.P., B.D.W., and S.G.D. contributed patient PBMC specimens, provided intellectual support, and gave critical advice on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael R. Betts, 402C Johnson Pavilion, 3610 Hamilton Walk, Philadelphia, PA 19104; e-mail: betts@mail.med.upenn.edu.