Platelets support the mobilization and migration of myeloid cells to distant sites undergoing active angiogenesis. Thus, platelet communication with the bone marrow facilitates a growing tumor's insatiable appetite for nutrients.
The relevance of myeloid cells (bone marrow–derived cells or BMDCs) and platelets to tumor growth has long been recognized but that both are mechanistically linked is the latest information that expands the relevance of platelets beyond their traditional role in hemostasis and thrombosis.1,2
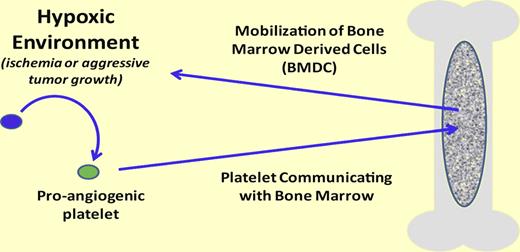
In the presence of a hypoxic environment (eg, ischemia or aggressive tumor growth) platelets become proangiogenic. Once proangiogenic, the platelet can release its cargo and actively interact/communicate with the bone marrow resulting in bone marrow–derived cell activation and recruitment. However, the mechanism controllong the release and/or control of the stimulation of bone marrow cell recruitment is unknown.
In the presence of a hypoxic environment (eg, ischemia or aggressive tumor growth) platelets become proangiogenic. Once proangiogenic, the platelet can release its cargo and actively interact/communicate with the bone marrow resulting in bone marrow–derived cell activation and recruitment. However, the mechanism controllong the release and/or control of the stimulation of bone marrow cell recruitment is unknown.
The late Judah Folkman pioneered the idea that growing solid tumors required active angiogenesis and methods to prevent angiogenesis potentially slowed, or even stopped, tumor growth by starving the tumor.3 Later, Folkman would implicate the involvement of the circulating blood platelet in angiogenesis supported by findings that platelets are a ready source of many proangiogenic proteins including vascular endothelial growth factor (VEGF).4 However, as is the case in many biologic pathways, for every profactor there exist antifactors. This, too, is the case with platelets, as they contain and may even “selectively” release both pro- and antiangiogenic factors.5 Yet to date, the release of these factors has been thought to operate solely within the hypoxic environment where new vessel growth is required (see figure).
In a separate line of investigation, BMDCs were found to be incorporated into newly formed blood vessels.6 These BMDCs home to the required area and differentiate into endothelial cells supporting angiogenesis. However, the mechanisms that trigger mobilization of the BMDCs or homing to an area where it is needed, as opposed to an area where it is not needed, were unknown.
In this issue, Feng and colleagues elegantly establish the interrelationship between circulating platelets and the BMDCs using a series of mouse models of platelet dysfunction.2 Platelet infusion promotes BMDC mobilization into the circulation where the BMDC ultimately finds a neovascularization-friendly environment. The use of distinct genetic mouse models confirms that the platelet function necessary for BMDC mobilization is secretion from the platelet's α granule compartment. Mice deficient in endobrevin (VAMP-8) have lost their ability to release α granule components and these platelets are unable to communicate with the bone marrow and signal BMDC mobilization. Interestingly, secretion associated with platelet dense granules or the presence of the platelet β 3 integrin receptor is not required to mobilize the BMDCs.
The data establish the important function of platelets using 2 distinct models of angiogenesis. The first is an aggressive solid tumor (melanoma) requiring an increased blood supply to support growth. The second is an experimental model of hypoxia that mimics is-chemic conditions, such as those associated with heart damage or retinopathies. Together, both animal models illustrate the mechanism by which a hypoxic environment uses the platelet to signal to the bone marrow and mobilize existing precursors to become mature endothelial cells.
Still unknown in this scenario is how the balance of the platelet's robust pro- and antiangiogenic abilities is controlled. However, the fact that platelet antiangiogenic properties are central is demonstrated in a mouse model lacking the key platelet antiangiogenic factor, thrombospondin-1.2 In the absence ofthrombospondin-1 the mobilization of BMDCs, vascularization, and tumor growth are all accelerated, establishing that the balance of the platelet's pro- and antiangiogenic factors is physiologically relevant. Thrombospondin-1 and VEGF are both components of platelet α granules and raises the intriguing possibility of selective platelet release, or the release of specific levels of pro- and antiangiogenic factors as needed. We might speculate that in the hypoxic tumor microenvironment the tumor cell is “preloading” the platelet with proteins needed to support its proangiogenic potential; perhaps even creating a proangiogenic bias. The platelet subsequently releases these factors that ultimately find their way to the bone marrow mobilizing a key component for the new vasculature.
Perhaps most relevant in these findings is the identification of platelet pathways which, if inhibited, would have a profound antiangio-genic outcome. Beta 3 integrin seems irrel-evant in this regard as do components associated with platelet dense bodies. The antiangiogenic effect of thrombospondin-1 is further documented and continues to show potential.7 Alpha granule release is clearly important for BMDC mobilization but what risk does blocking α granule release pose for normal hemostasis? These are all areas for future investigation aided by the demonstration of platelet communication with the myeloid microenvironment and the influence on angiogenic pathways. Clearly, the biologic role of the platelet has expanded well beyond hemostasis and thrombosis.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal